Prodigest®
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionPeer-reviewed science on PRODIGEST®
Prodigest® facilitates gastric emptying in a dose-dependent manner.
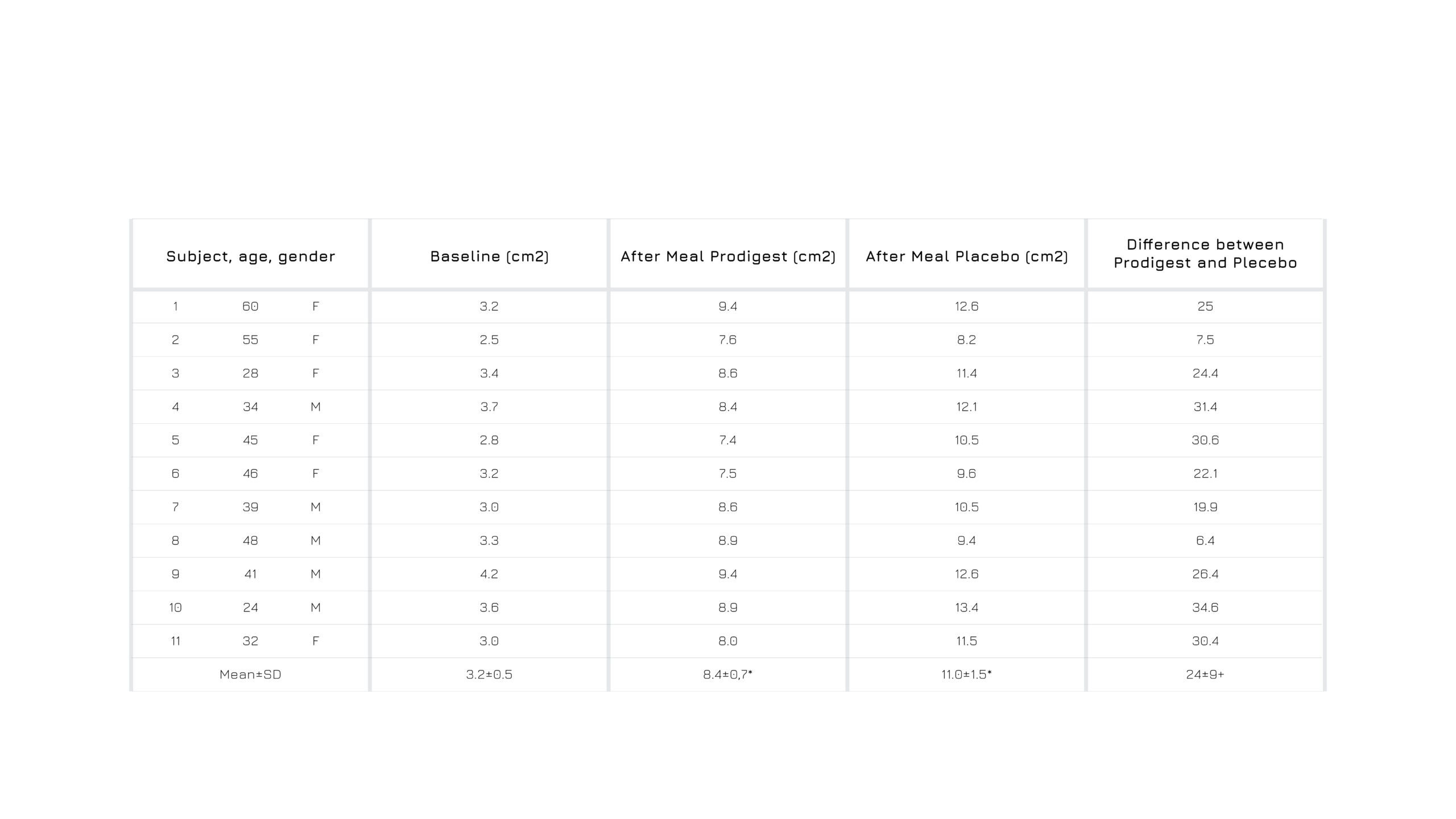
After a meal administered with Prodigest®, the after-meal gastric area of 11 volunteers is significantly smaller in the administrated group (-24%) compared with the not-administrated one (8.4 cm2 versus 11.0 cm2). Further, with a double dose of PRODIGEST®, the after-meal gastric area is 6.4 cm2.
Figure 1: Gastric area (cm2) variation, at baseline and after a meal administrated with one capsule of Prodigest® or not.
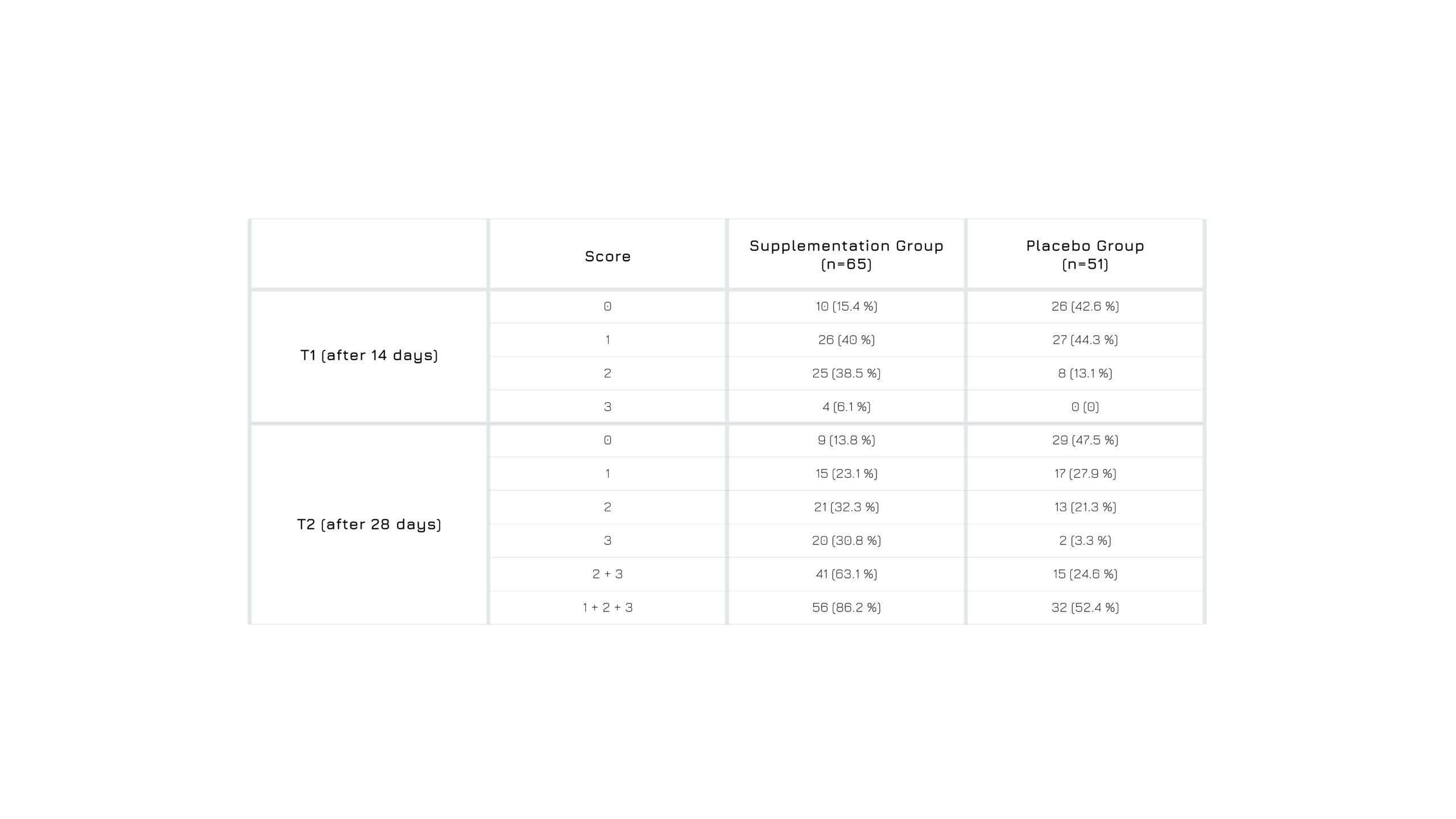
Prodigest® is safe and beneficial in digestive discomforts. A group of 65 people (18-70 years) with digestive discomforts receiving Prodigest® for 4 weeks shows a significant positive effect in 86.2% of cases, after 28 days of Prodigest® administration, with marked effect on digestive discomforts in 63.1% of the cases.
The statistical analysis demonstrate a significant effect on intensity score for epigastric stress over the observation time, too.
Figure 2: Variation of digestive discomforts intensity over time following Prodigest® administration or not.
BIBLIOGRAPHY
1Lazzini S., et al., Eur. Rev. Med. Pharmacol. Sci., 20: 146-149 (2016).
2Giacosa A. et al., Evidence Based-Complementary And Alternative Medicine, Volume (2015).
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.