CUBO™
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionPeer-reviewed science on CUBO™
A randomized, placebo-controlled study was conducted on 49 adults with an IBS or small bowel dysbiosis diagnosis. All subjects followed a low-FODMAP diet; 24 of them were also supplemented with a 380-mg tablet of CUBO™, twice a day, while the rest formed a control group and received a placebo.1
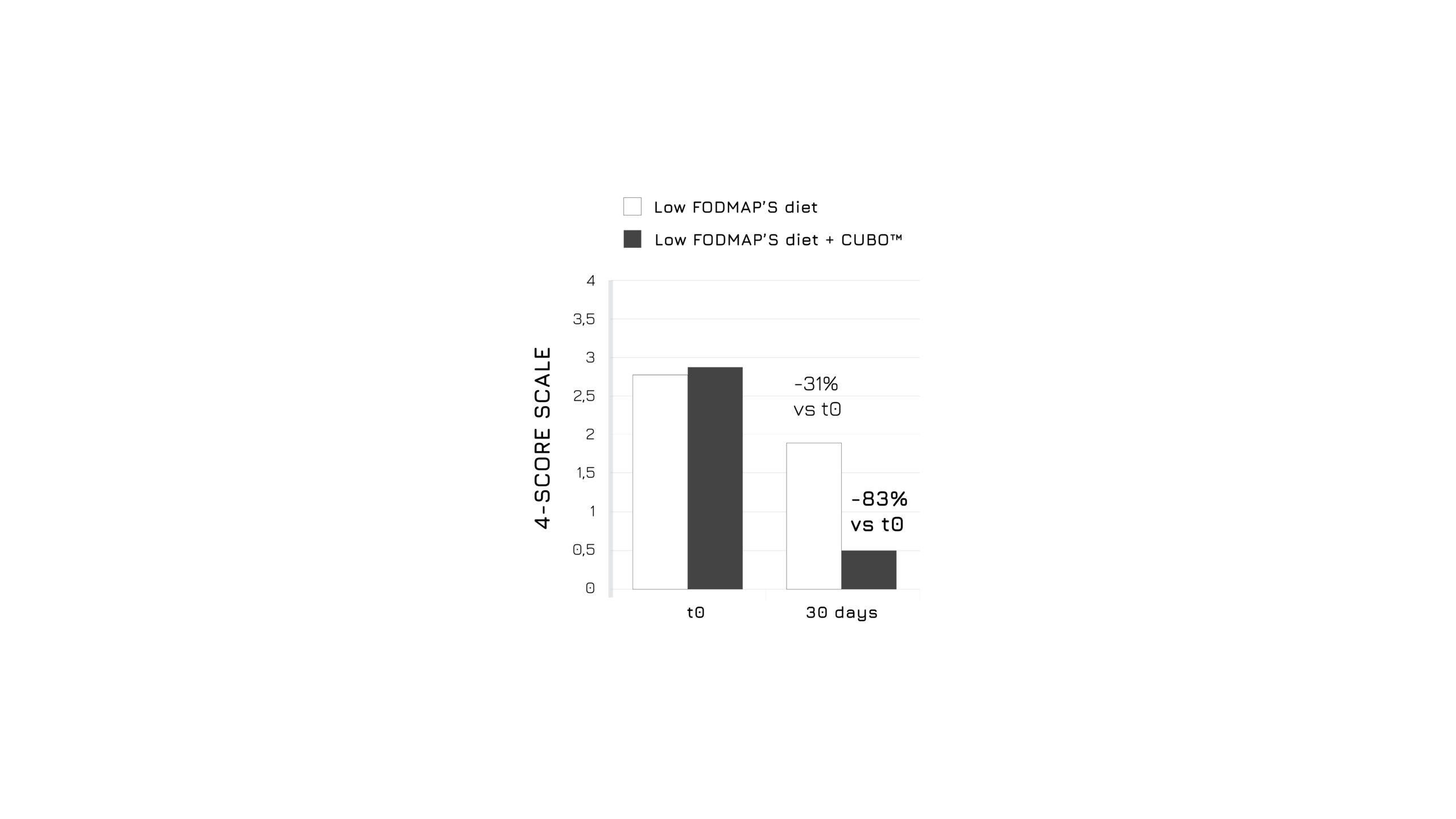
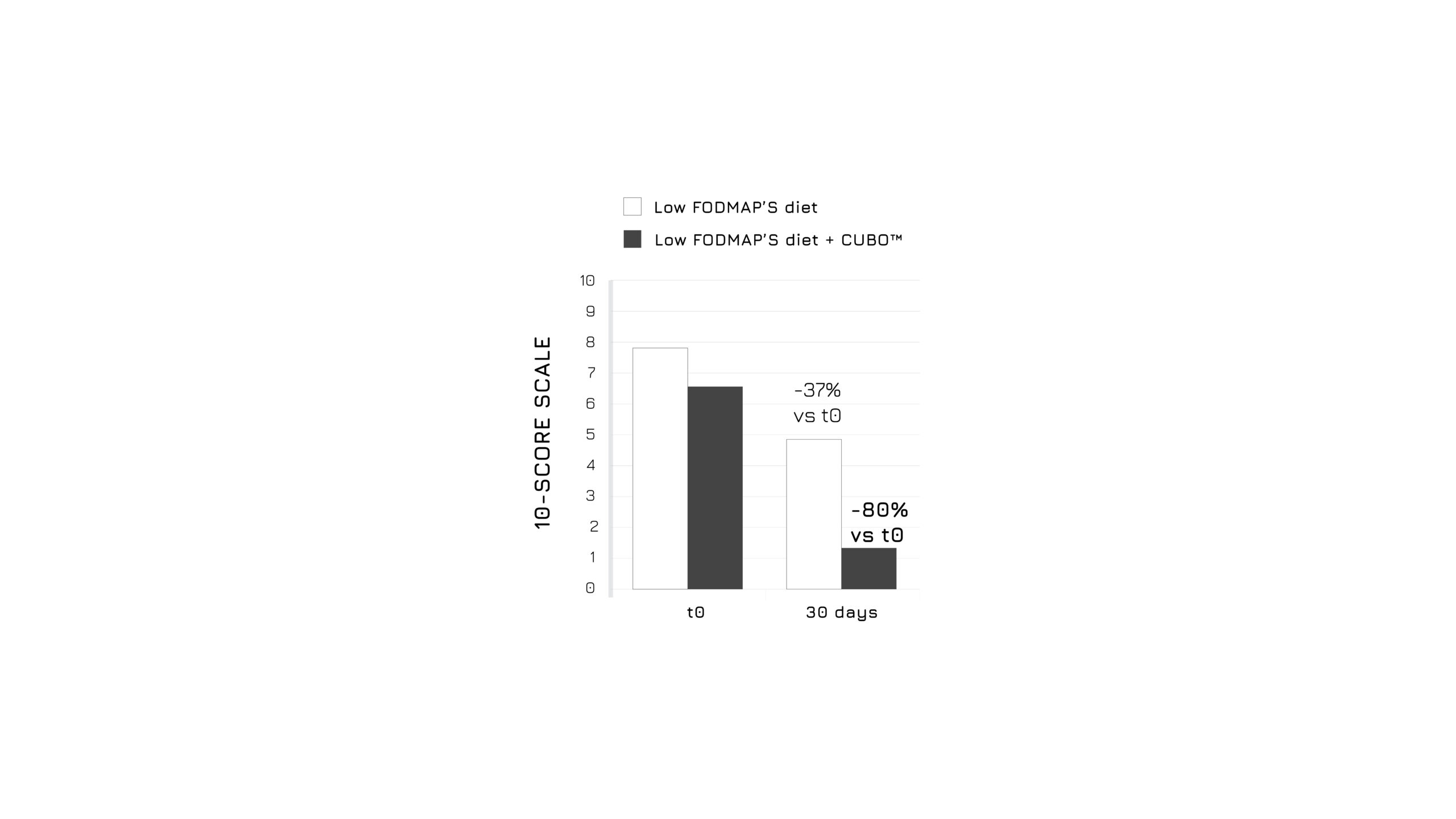
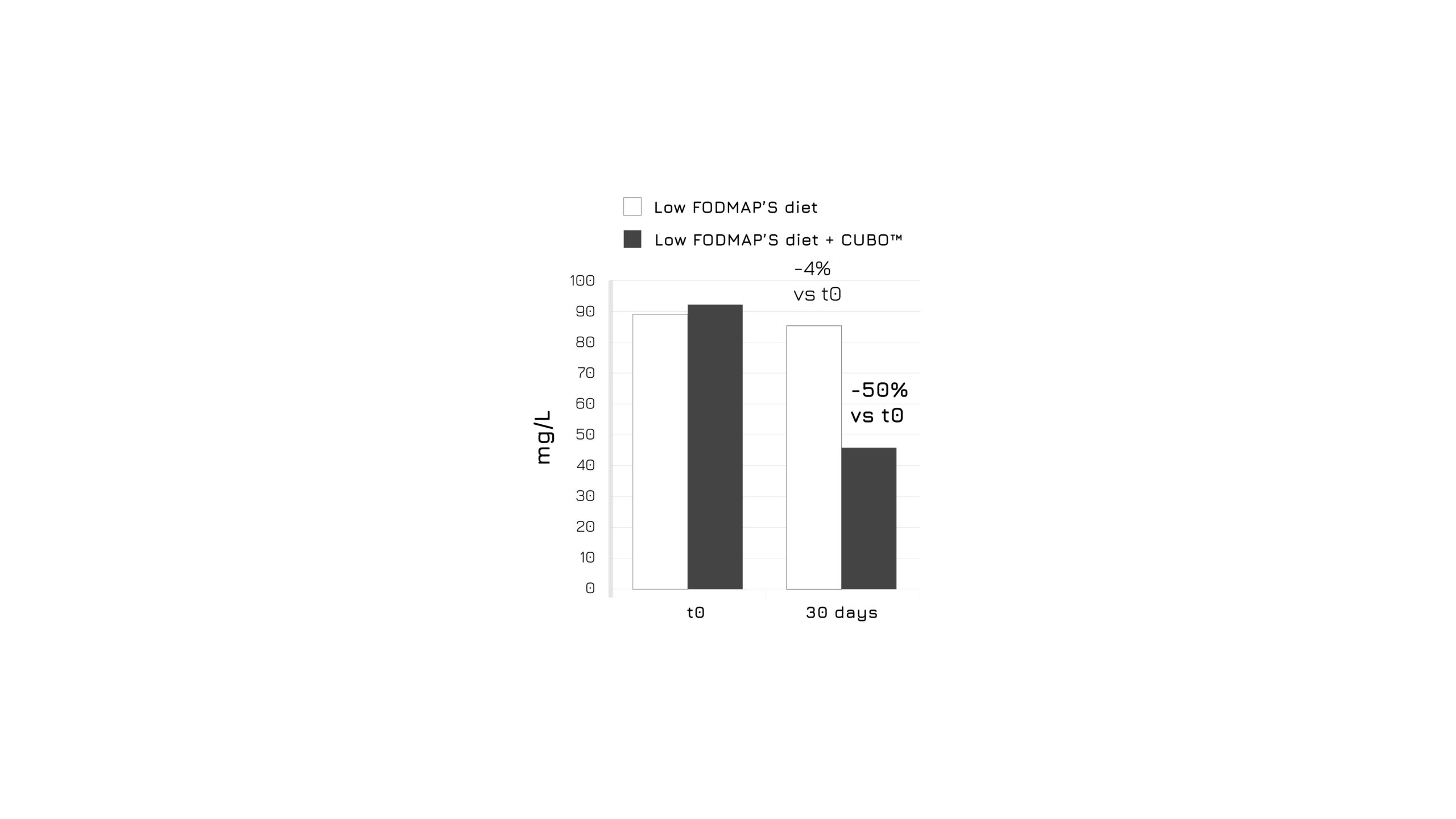
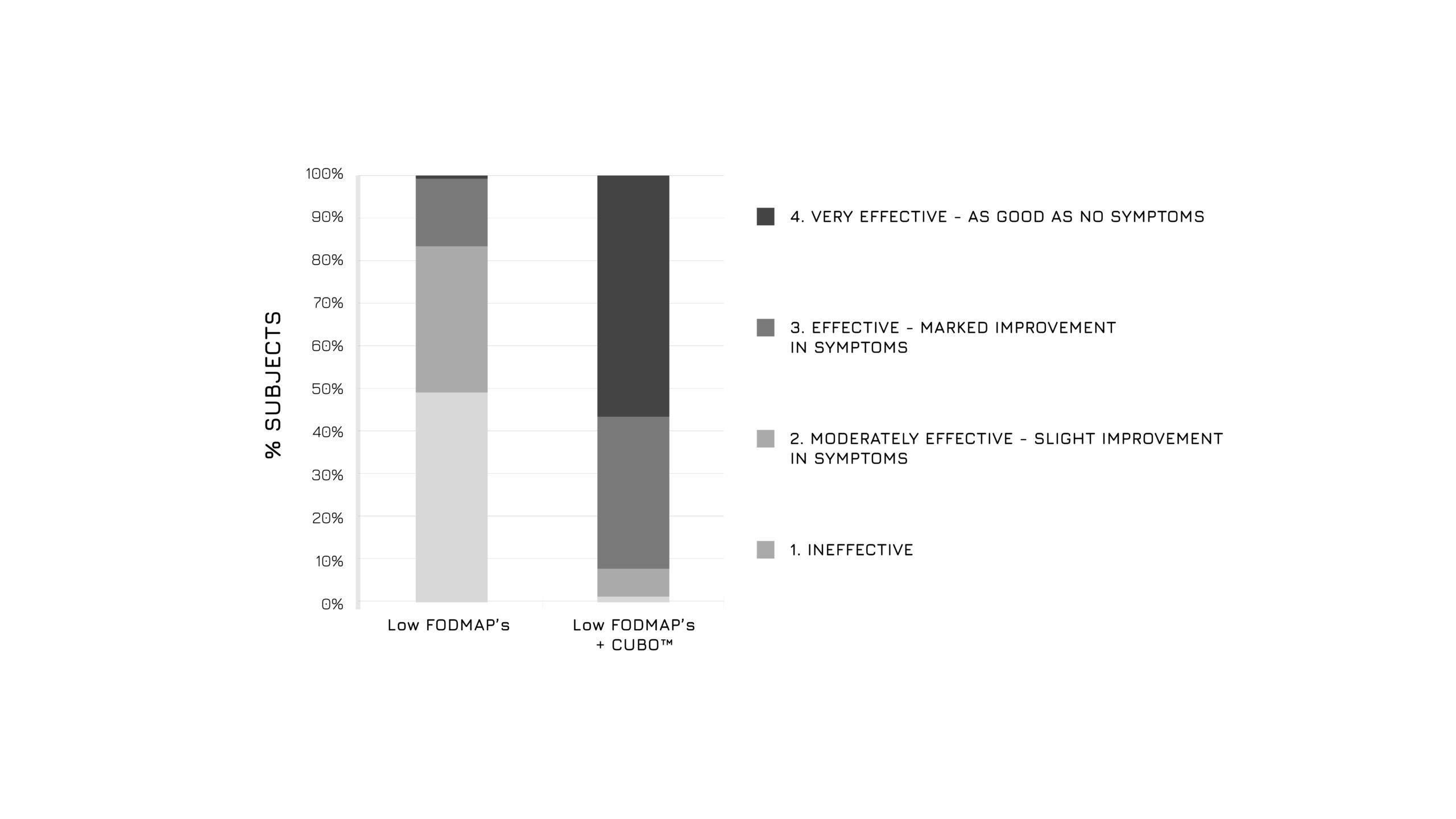
After 30 days of admininistration, the study showed a significant bloating control in the supplemented group (-83%, p<0.0001), as assessed using a 4-score questionnaire (Figure 1). Moreover, authors highlighted a relevant beneficial effect in abdominal discomfort control (Figure 2) and in intestinal dysbiosis markers (Figure 3). Overall, the global assessment of efficacy (GAE) showed that 91.6% of supplemented subjects reported a high efficacy (Figure 4).1
Figure 1: Graphical representation of the bloating intensity modulation in control and supplemented groups after 30 days. P<0.0001, model adjusted for sex and age.
Figure 2: Graphical representation of the abdominal discomfort modulation in control and supplemented groups after 30 days. P<0.0001, model adjusted for sex and age.
Figure 3: Graphical representation of urinary markers for intestinal dysbiosis in control and supplemented groups after 30 days. P<0.0001, model adjusted for sex and age. Levels of a metabolic product, resulting from bacterial action on tryptophan in the small bowel, were used as a marker of dysbiosis and malabsorption.
Figure 4: Graphical representation of the Global Assessment of Efficacy (GAE) in control and supplemented groups after 30 days.
BIBLIOGRAPHY
1. Data to be published.
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.