Enovita®
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionEnovita® and brain health
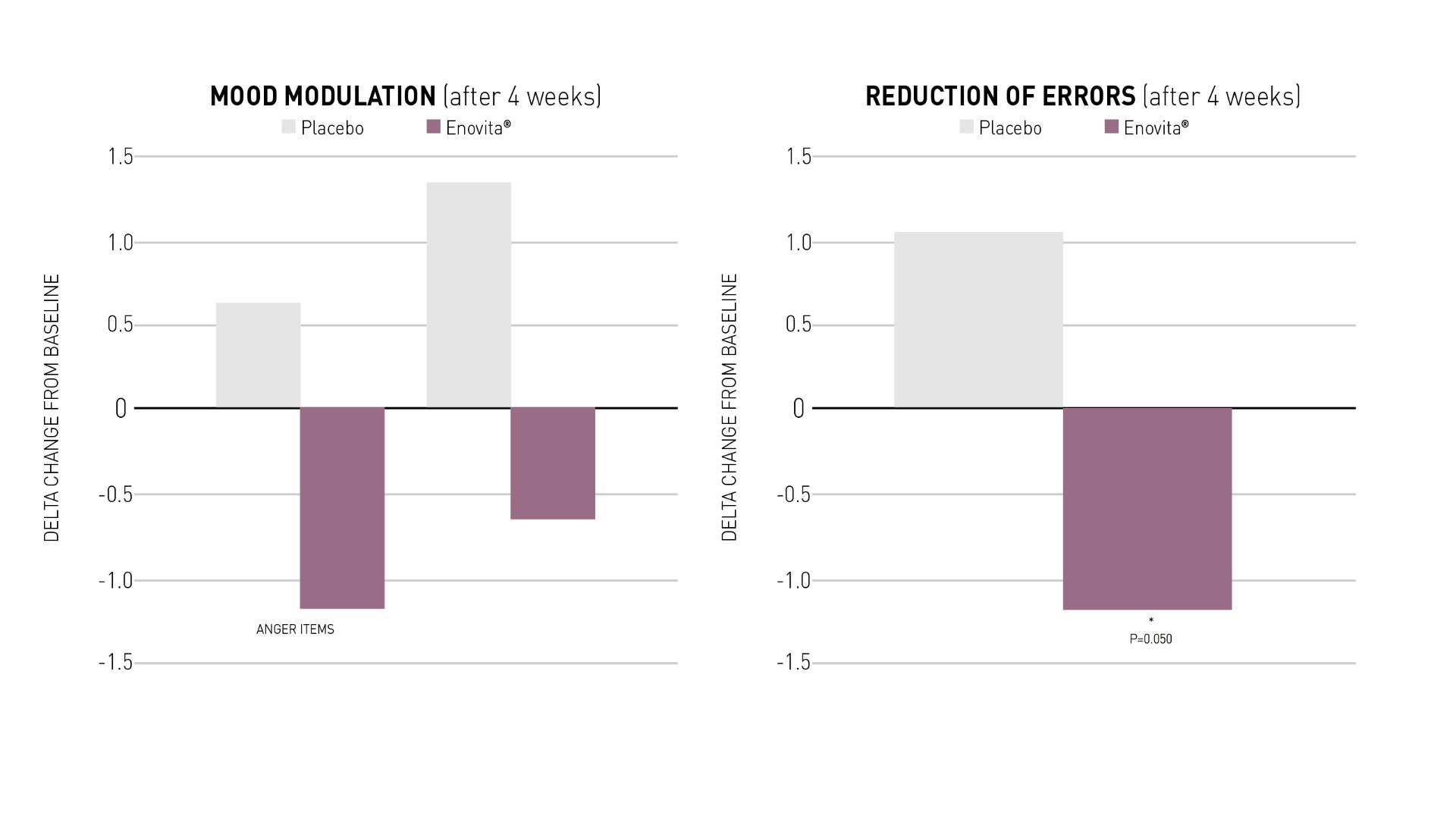
The capacity to regulate mood and enhance overall well-being plays a vital role in improving quality of life and daily functioning.
Prolonged supplementation with Enovita® has shown a beneficial effect on mood modulation as assessed by POMS test (Profile Of Mood States), particularly in reducing feelings of anger and mitigating symptoms related to depression.
As a positive impact on emotional balance can contribute to a more stable and improved mood state over time, conversely a bad mood could be linked to errors in remembering short-term information, translating into major errors in everyday calculations and then affecting decision-making.
Enovita® 4-week supplementation has shown statistically positive effects on working memory and on errors reduction, i.e. improving how well individuals can perceive and accurately attend to short-term symbols and stimuli, as measured by a 4-part continuous task.
Peer-reviewed science on Enovita®
A recent study on Enovita® included 80 healthy participants that were randomized to receive GSE supplementation or placebo twice a day for a total of 16 weeks. The principal endpoints included pressure modulation and the perception of stress and worries.1
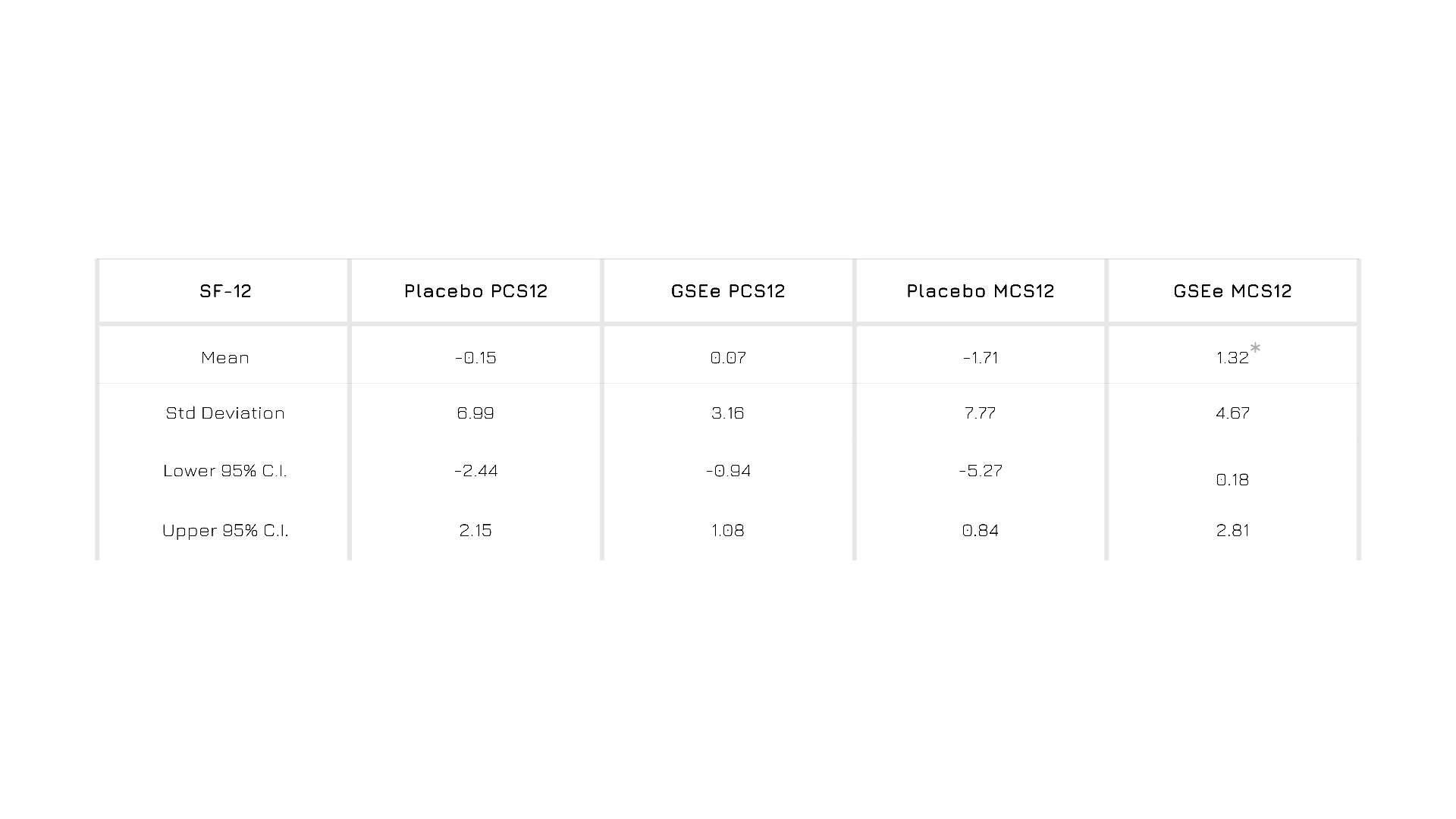
Enovita® supplementation is associated with a general increase of mental health and quality of life (quality of life questionnaire SF-12 and mental component summary MCS12).1
Figure 1. Results from SF-12 self-administered questionnaire.
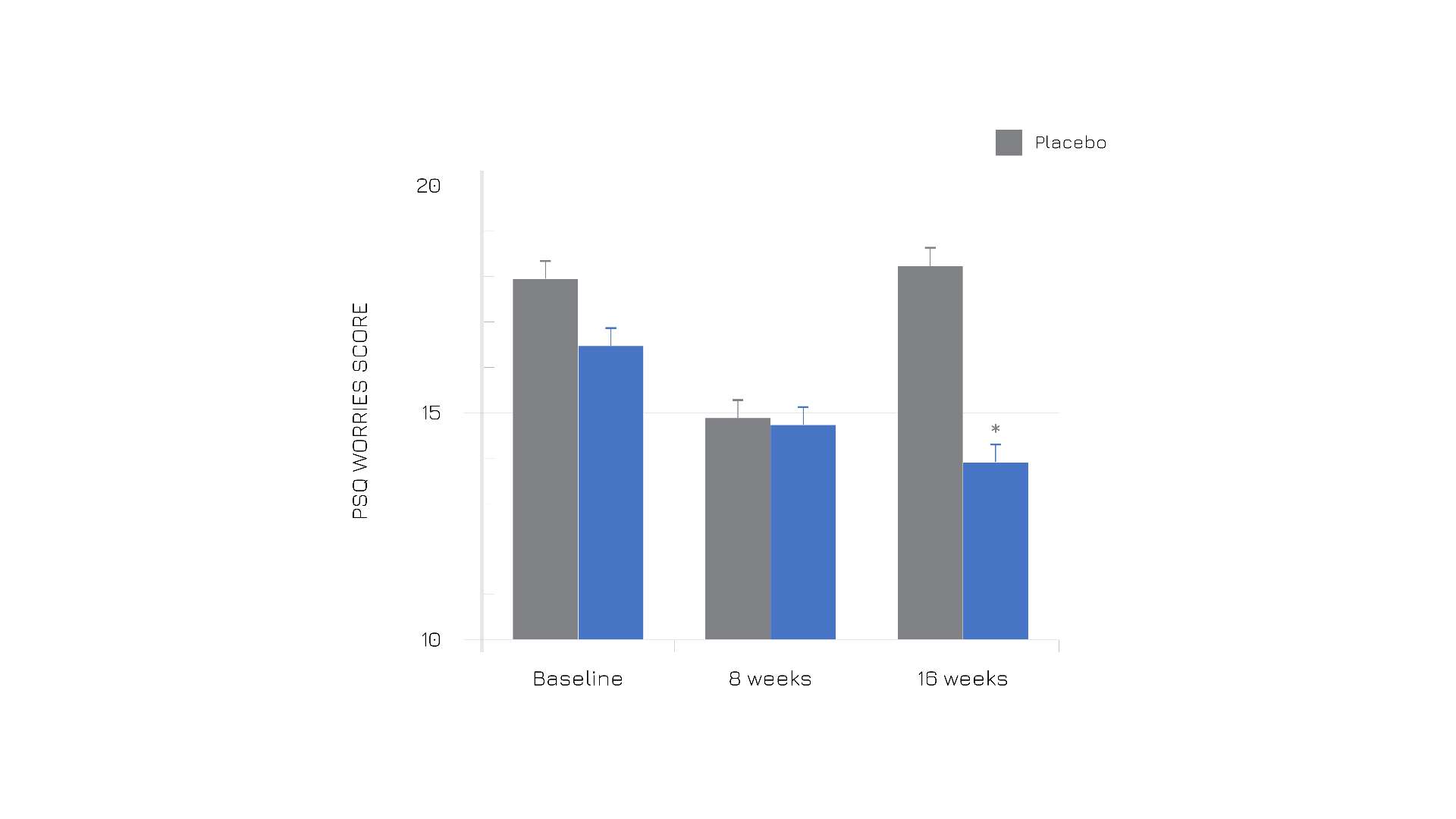
The perception of worries under Enovita® supplementation was less pronounced (Figure 2).
Figure 2. Results from PSQ self-administered questionnaire at baseline, after 8 and after 16 weeks. (Validated tool to assess subjectively experienced stress independent of a specific or objective occasion).Graphic elaboration of table 2, from ref. 1.
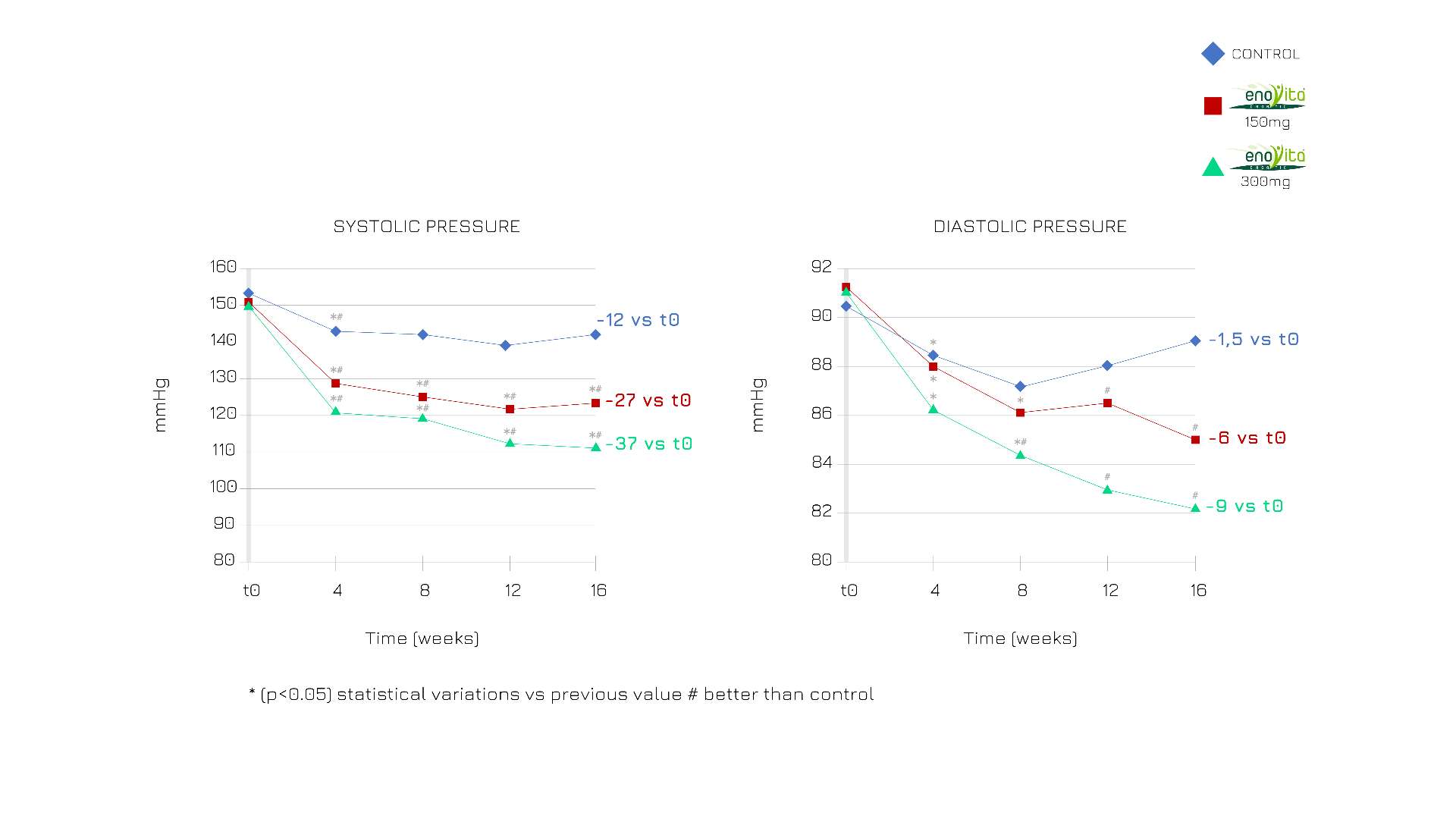
Enovita® significantly modulated both systolic and diastolic pressure with a dose-dependent effect.1
Figure 3. A favorable effect of systolic blood pressure was observed in all groups, but the effect was significantly higher in the treatment group (p < 0.05). The effect was lower for diastolic pressure. Thus, in the two treatment groups, an effect on diastolic pressure was also observed and developed more gradually over time.1
Enovita® can have beneficial cardiovascular effects that may support intervention strategies in conditions of cardiac stress.
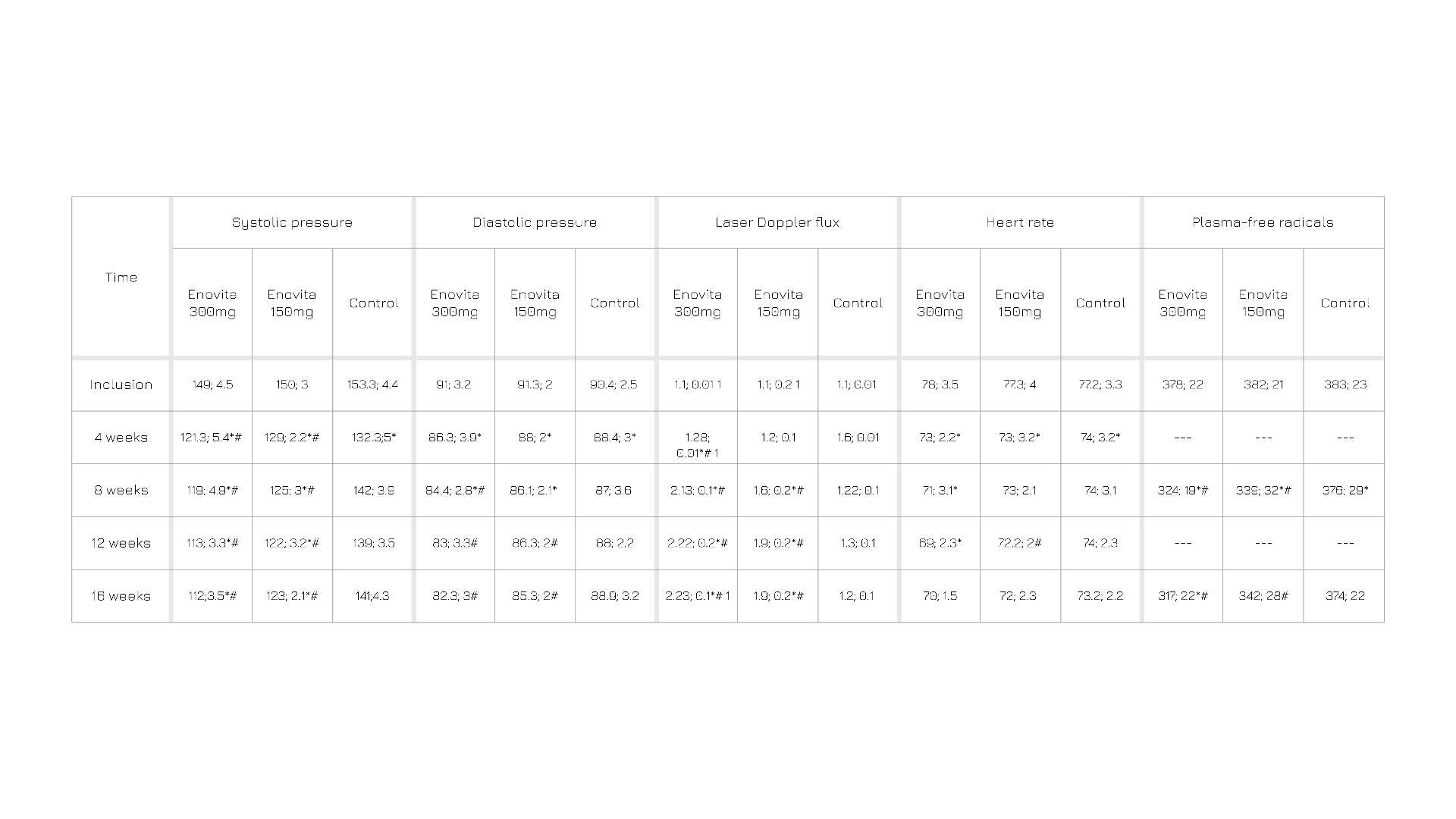
In a human study, after 4 months of administration, a statistically significant and dose-dependent improvement in all endpoints (systolic pressure, diastolic pressure, laser doppler flux, heart rate, plasma-free radicals) was observed in both groups delivered with Enovita® compared to the one not receiving supplementation, with blood pressure normalizing in 93% of the higher dosage (300 mg) administrated group. Altogether, these observations suggest that Enovita® has beneficial cardiovascular effects that may support current management strategies against hypertension.
Table 1. Systolic pressure, diastolic pressure, laser doppler flux, heart rate, plasma-free radicals in the two groups administrated with Enovita® (300 mg and 150 mg) and in the one not administrated, at inclusion, 4, 8, 12, and 16 weeks.
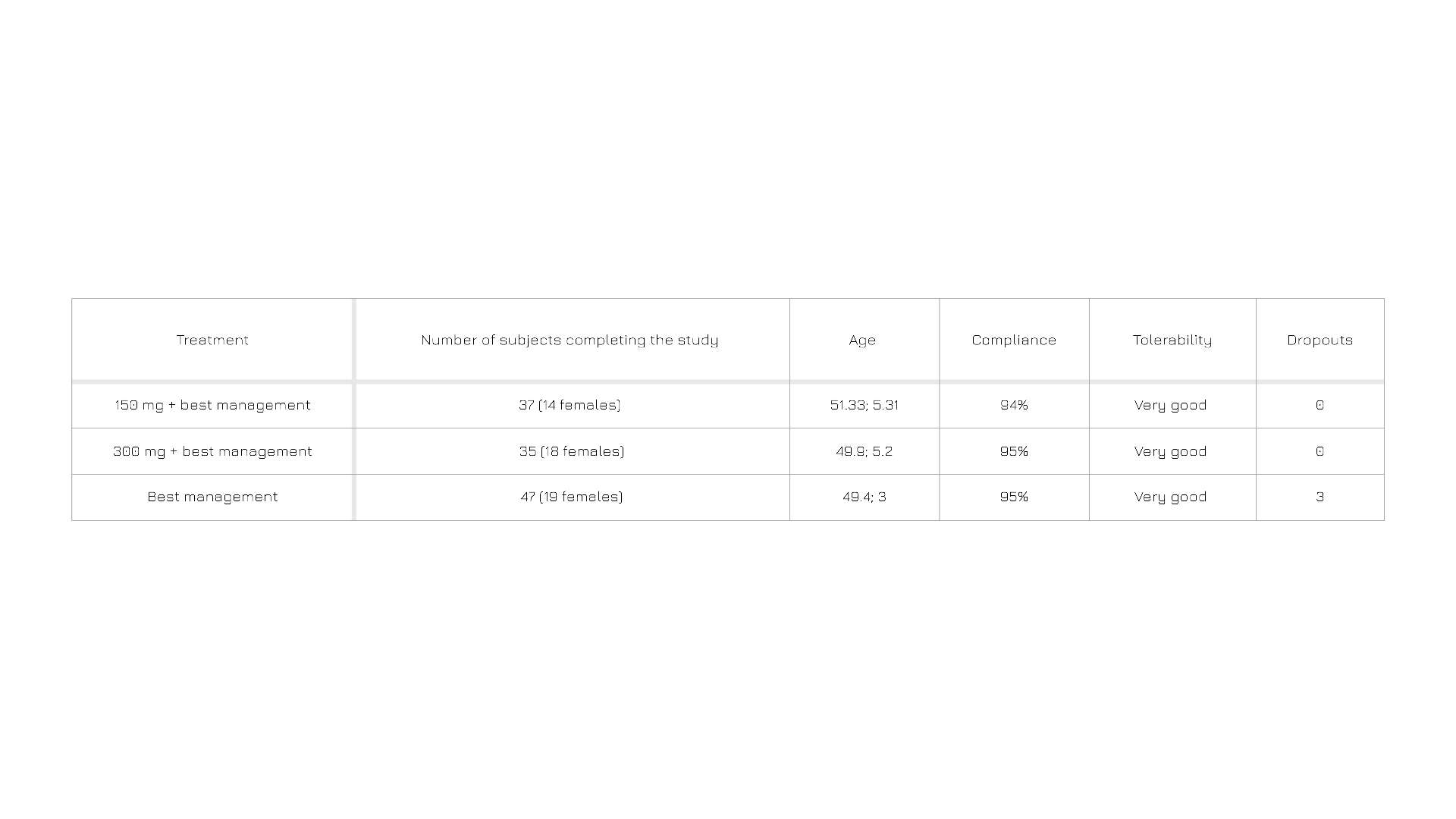
Table 2. Subject compliance and tolerability at the end of the trial.
BIBLIOGRAPHY
1Schön C, et al. Nutrients. 13(2):654. (2021)
2Belcaro G., Ledda A., Hu S., et al., Evidence-Based Complementary and Alternative Medicine, ID 313142 (2013).
3Confidential data on file. Manuscript in preparation (2024).
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.