Meriva® – Curcumin Indena Phytosome™
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionPeer-reviewed science on Meriva®
Meriva® sports an outstanding pre-clinical and clinical evidence.
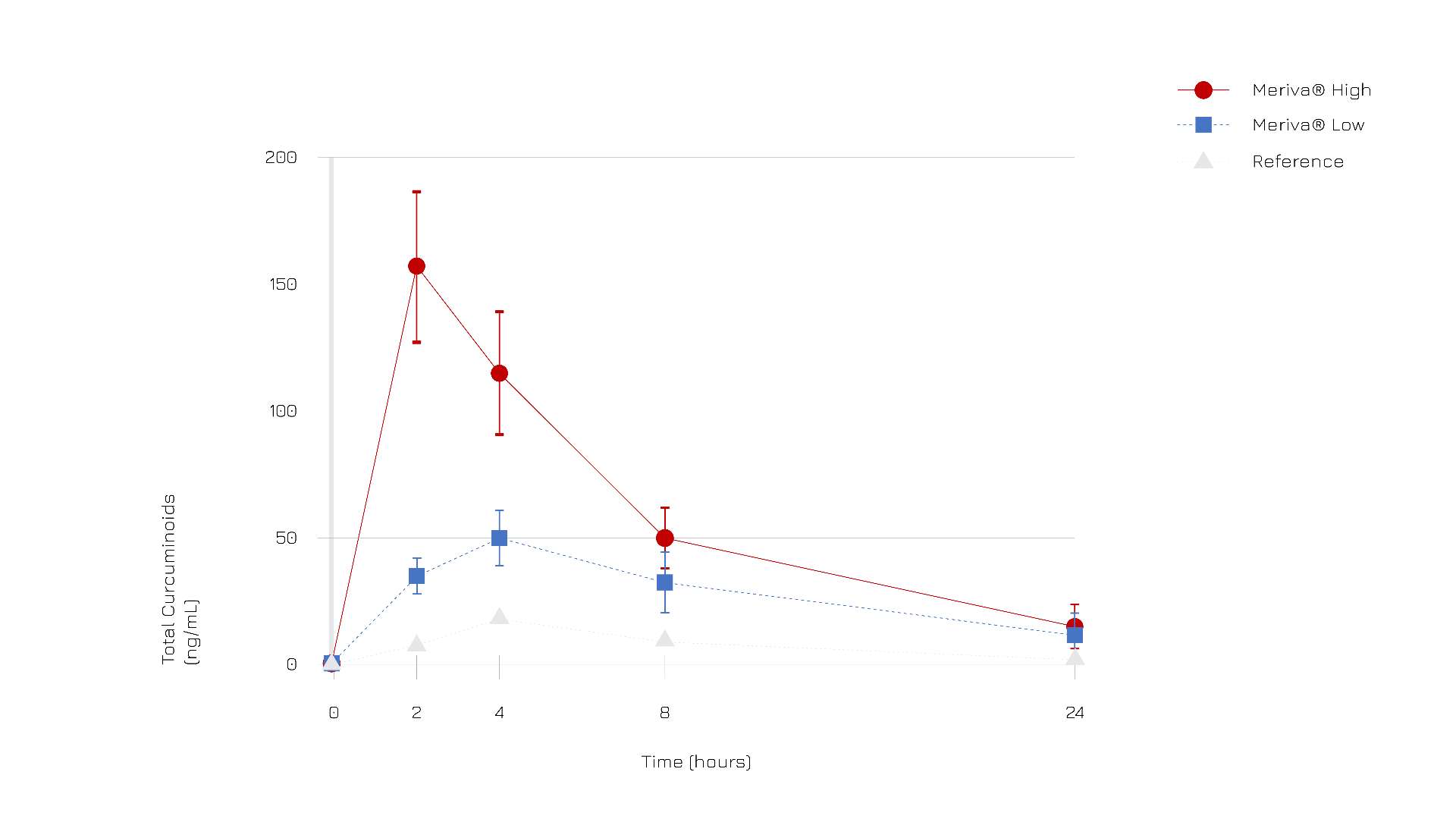
According to clinical evidence comparing the pharmacokinetics profile of Meriva® and a reference standard turmeric extract, curcuminoids bioabsorption was about 30-fold higher for Meriva® than for its corresponding unformulated curcuminoid mixture (Fig.1).
More specifically, Meriva® demonstrated to optimize the bioabsorption of all three curcuminoids, in particular DMC (demethoxy curcumin) which is known for its powerful activity.
Figure 1: Total curcuminoids (ng/mL) absorption profile after oral administration of Meriva® (high dose, dots; low dose, squares) and reference standard (triangles).
Meriva® at a daily dose of 2 g/day, for up to 18 months, hasn't caused any discomfort or significant side effects on all participants, thus supporting a long term supplementation.
Meriva® plays a key role in supporting the musculoskeletal system, particularly in conditions associated with natural aging process, chronic discomforts or external factors (such as certain pharmacological treatments). This potent natural ingredient has shown positive effects in maintaining joint, bone, and muscle health, improving both physical performance and quality of life.
Joint Health
Meriva® has been demonstrated to support effective ache management, with a 58% optimization versus standard approaches according to the WOMAC scale. Physical performance improved by 26% (Karnofsky scale), joint mobility by 4-fold on treadmill test, and overall quality of life by 3-fold (Social and Emotional Index). Additionally it also aids in optimizing inflammatory biomarkers, with a 65% decrease in interleukin 1-beta and a significant 16-fold reduction in CRP levels. Furthermore, subjects reported reduced need for additional support.
Bone Health
Meriva® has been linked to optimized bone density, particularly in key areas such as the heel, small finger, and upper jaw. This supports better structural integrity, especially in ageing populations.
Muscle Health
Meriva® supplementation has shown marked benefits in muscle performance, particularly in silver age. After three months of supplementation, studies showed a 9% increase in handgrip strength, a 23% improvement in weight lifting capacity, a 24% boost in walking distance, and a 39% enhancement in stair-climbing performance, all compared to baseline.
Meriva®’s multi-faceted role in supporting joint, bone, and muscle health highlights its value as a natural supplement for improving mobility, strength, and overall well-being.
The use of Meriva® in addition to standard management, provided increased relief to atopic-prone skins and inflammation-based discomforts, improving parameters like itch, drieness, sensitivity, warming sensation and exfoliation.
Furthermore, a statistically significat optimization of healthy inflammatory biomarkers has been demonstrated.
Low grade inflammation and oxidative stress are recognized among the disrupting factor for renal functionality and kidney health. In two human studies Meriva® have been tested to verify its potential to maintain normal and physiological renal values in subjects with diverse risk factors.
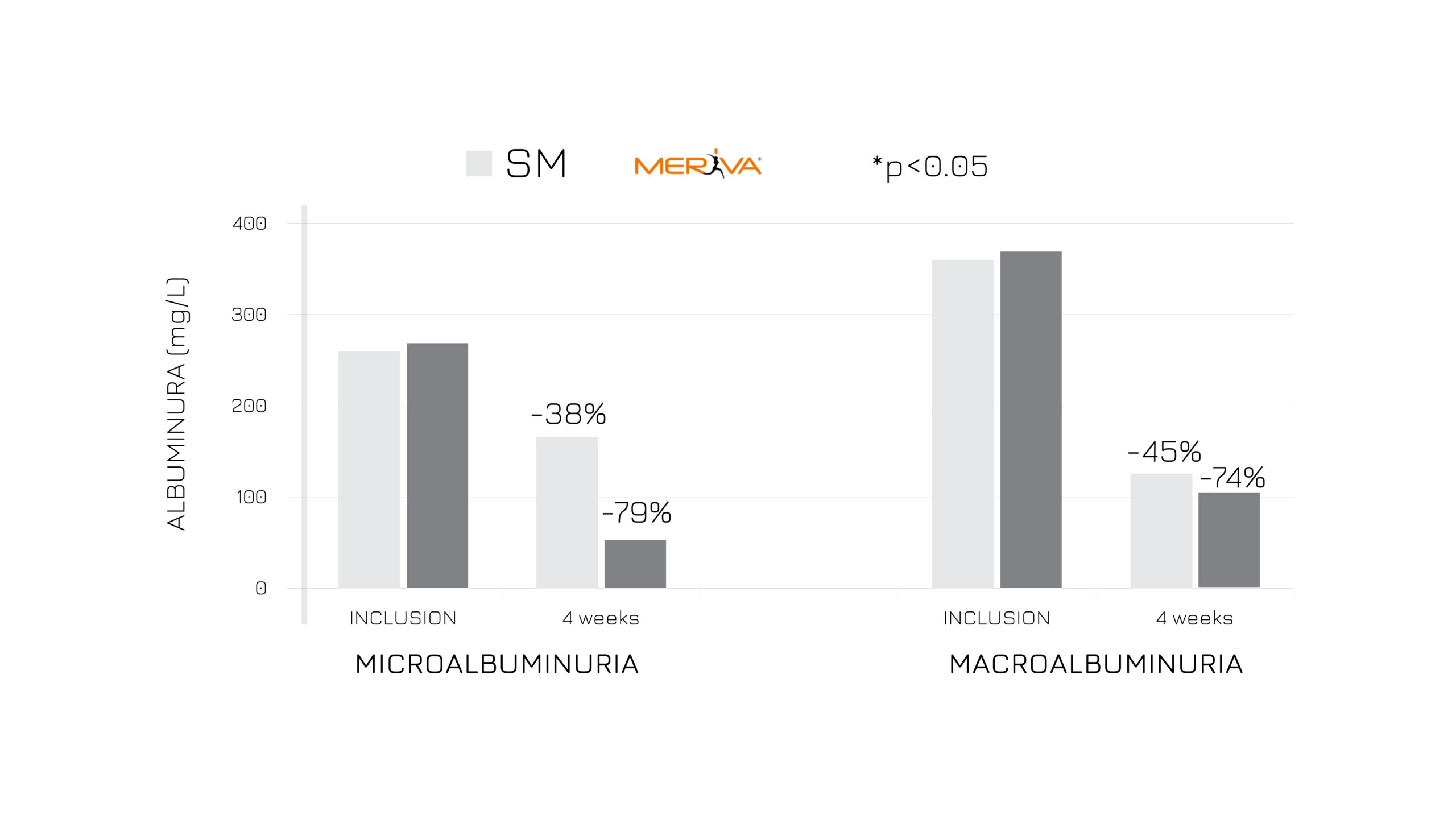
The first study has been conducted on 87 asymptomatic subjects with temporary altered kidney parameters. The ancillary supplementation of 1.5 g/day of Meriva® positively influenced albuminuria, oxidative stress and fatigue after 4 weeks compared to the standard management (Fig.2).
Furthermore, a study showed that Meriva® supplementation (1.0g/day) is associated to the amelioration of healthy inflammatory response and oxidative balance in subjects (n=24) with mildly altered kidney function.
Figure 2: Albuminuria (mg/L) in subjects treated with SM vs SM+Meriva® at V0 and after 4 weeks, considering microalbuminuria and macroalbuminuria values.
BIBLIOGRAPHY
1Cuomo J. et al., J Nat Prod. Mar 17. (2011).
2Musso G. et al., Hepaplogy, May 28 (2024).
3Belcaro G. et al., Altern Med Rev 15(4):337-44 (2010).
4Belcaro G. et al., Panminerva Med 52(2Suppl1):55-62 (2010)
5Belcaro G. et al., Eur Rev Med Pharmacol Sci 18(24)3959-63 (2014).
6Riva A. et al., Eur Rev Med Pharmacol Sci 21(7):1684-89 (2017).
7Franceschi F. et al., Eur Rev Med Pharmacol Sci. 20(4):762-66 (2016).
8Togni S. et al., Dermatological Experiences 21(2-4):42-6 (2019)
9Antiga E. et al., Biomed Res Int 2015.1: 283634 (2015)
10Ledda A. et al., Panminerva Medica.Dec 61(4):444-48 (2019).
11Pivari F. et al., Nutrients,.Jan 14 231 (2022)
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.