Casperome®
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionPeer-reviewed science on Casperome®
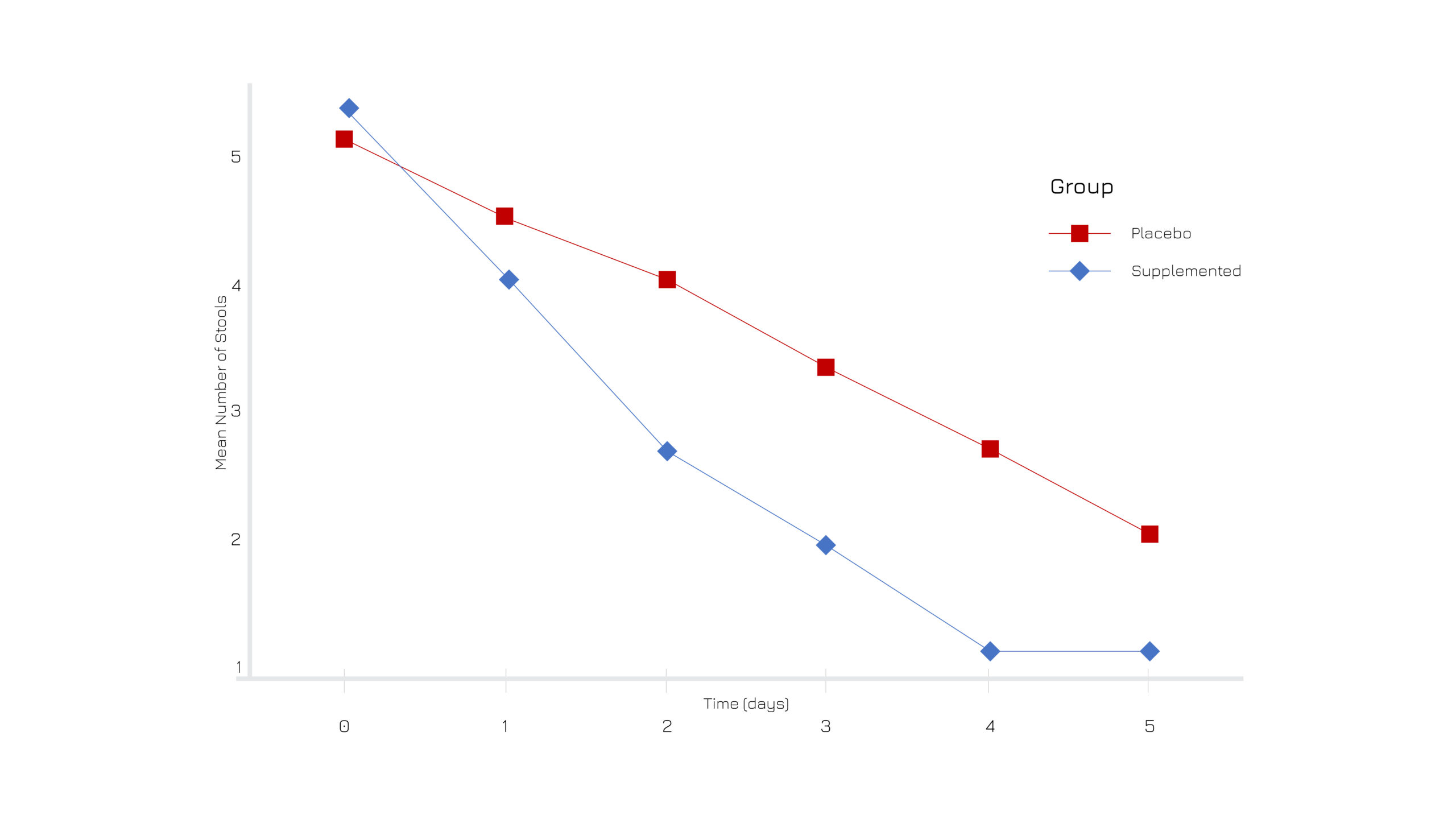
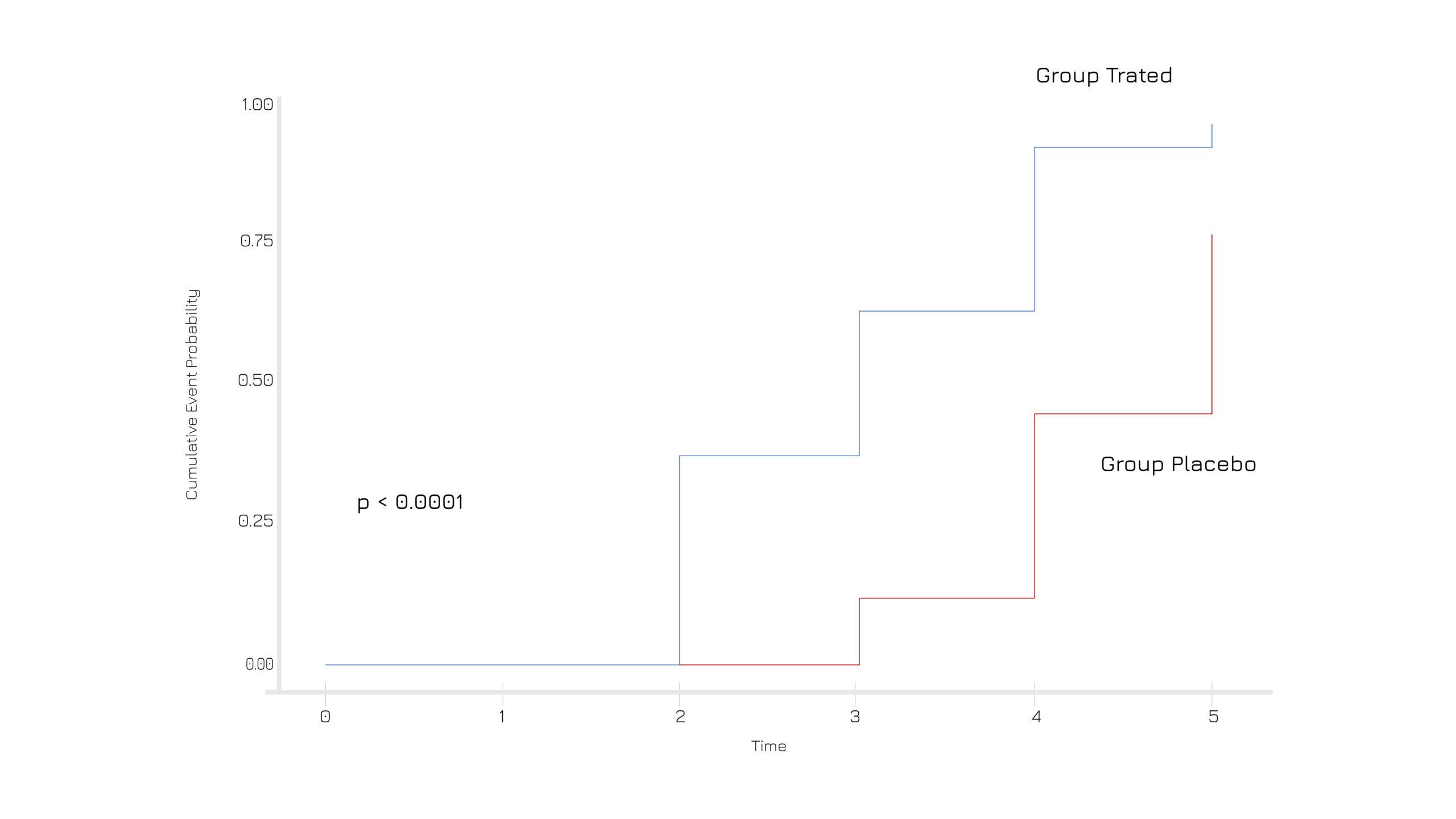
A randomized, double-blind, placebo-controlled trial on 49 adults with acute diarrhea administered with 250 mg of Casperome® 2 times per day, showed a significant reduction frequency of stools and duration of diarrhea, along with associated secondary conditions (Figure 1). At the end of the study, the percentage of subjects free from diarrhea within 5 days of supplementation was 95.8% in the treated group vs 76% in the placebo (Figure 2). No side effects have been observed. Mechanistic evaluations also proved that the Boswellia serrata extract can act as a Ca2+ channels modulator on intestinal smooth muscles.1
Figure 1: Graphic representation of the mean number of stools over time.1
Figure 2: The log-rank test showed a median time of a 3 days of becoming healthy for the supplemented group and a median of 5 days for the placebo group.1
A study involved 43 participants with a story of Ulcerative Colitis that received an oral daily Casperome® supplementation (n = 22) or no supplementation (n = 21) for 4 weeks. The Casperome® group experienced overall better well-being feeling compared to the control, that required some treatment and medical consultations.
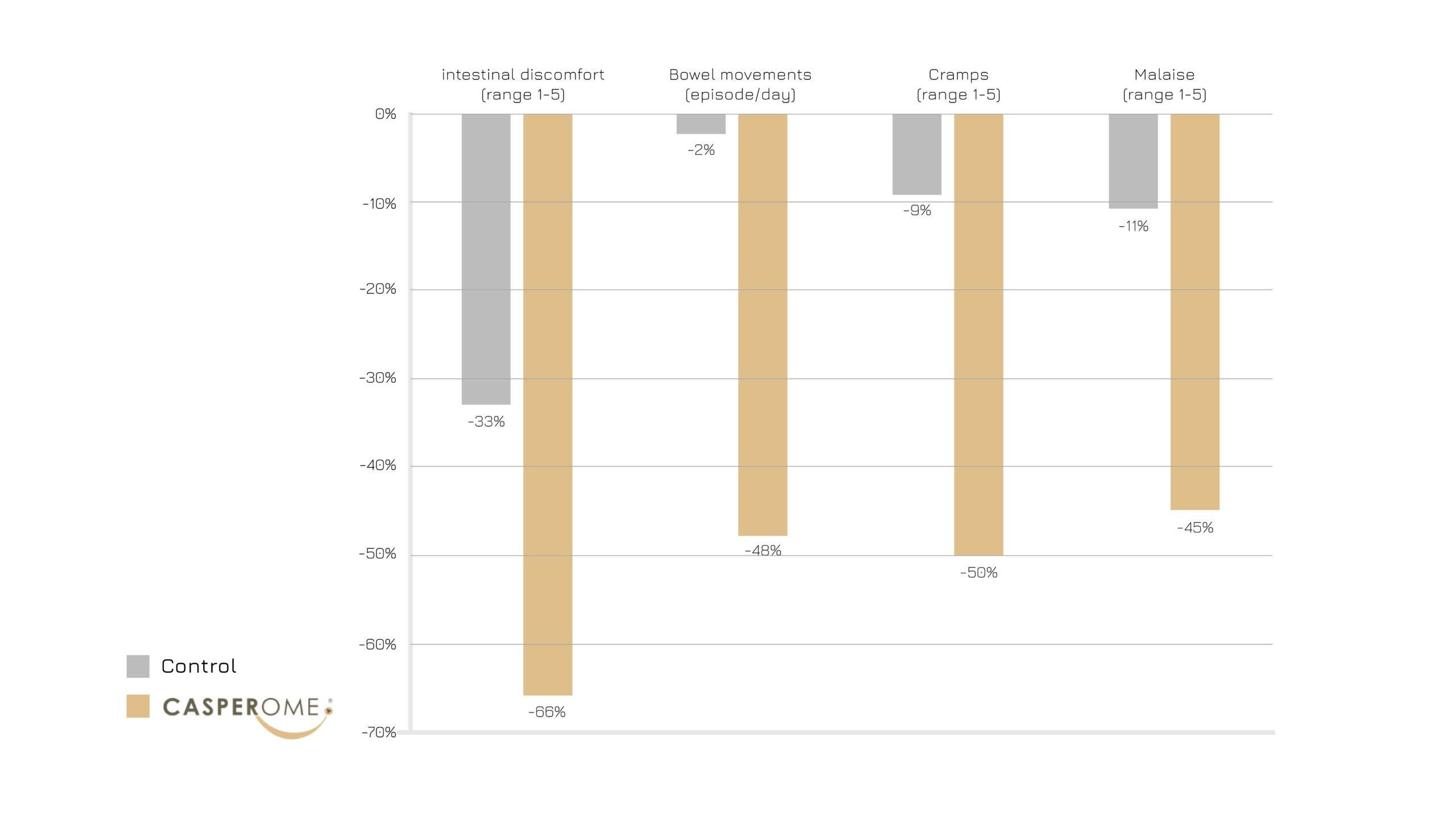
Figure 3: Evaluation criteria, observed before and at the end of the study.2
Another trial involved 71 healthy subjects with idiopathic gastrointestinal discomforts. The participants were divided into 3 groups and randomized with Hyoscine butylbromide (Buscopan®), Papaverine hydrochloride 10 mg + belladonna (A. belladonna) and Casperome®. The number of subjects experiencing persistent gastrointestinal well-being counted more after the observational period in Casperome® supplemented group (from 42% to 87.5%). Consistently, the number of subjects who needed medical attention significantly decreased to 4.1%.3
Finally, in a 6-month study on 69 participants, subjects on Casperome® showed lower mean score values, compared with the standard management group, for almost all output. The incidence of minimal adverse events was significantly higher in the standard management group.4
Buscopan® is a registered trademark of Sanofi SA.
Casperome® has beneficial effects in case of muscoskeletal and sport stress in general. All studies are consistent in showing a prompt decrease of discomforts and amelioration of functionality of the affected area after Casperome® administration, without relevant adverse events.
In a human study on young rugby players with acute knee stress, after a 4-week follow-up, only 6 subjects of those administrated with Casperome® show local stress vs. 25 at baseline. Moreover, the administrated group has significantly less need of other support compared to the standard-management group.5
PK OF CASPEROME® VS BASIC B. SERRATA EXTRACT DELIVERED BOSWELLIC ACIDS.
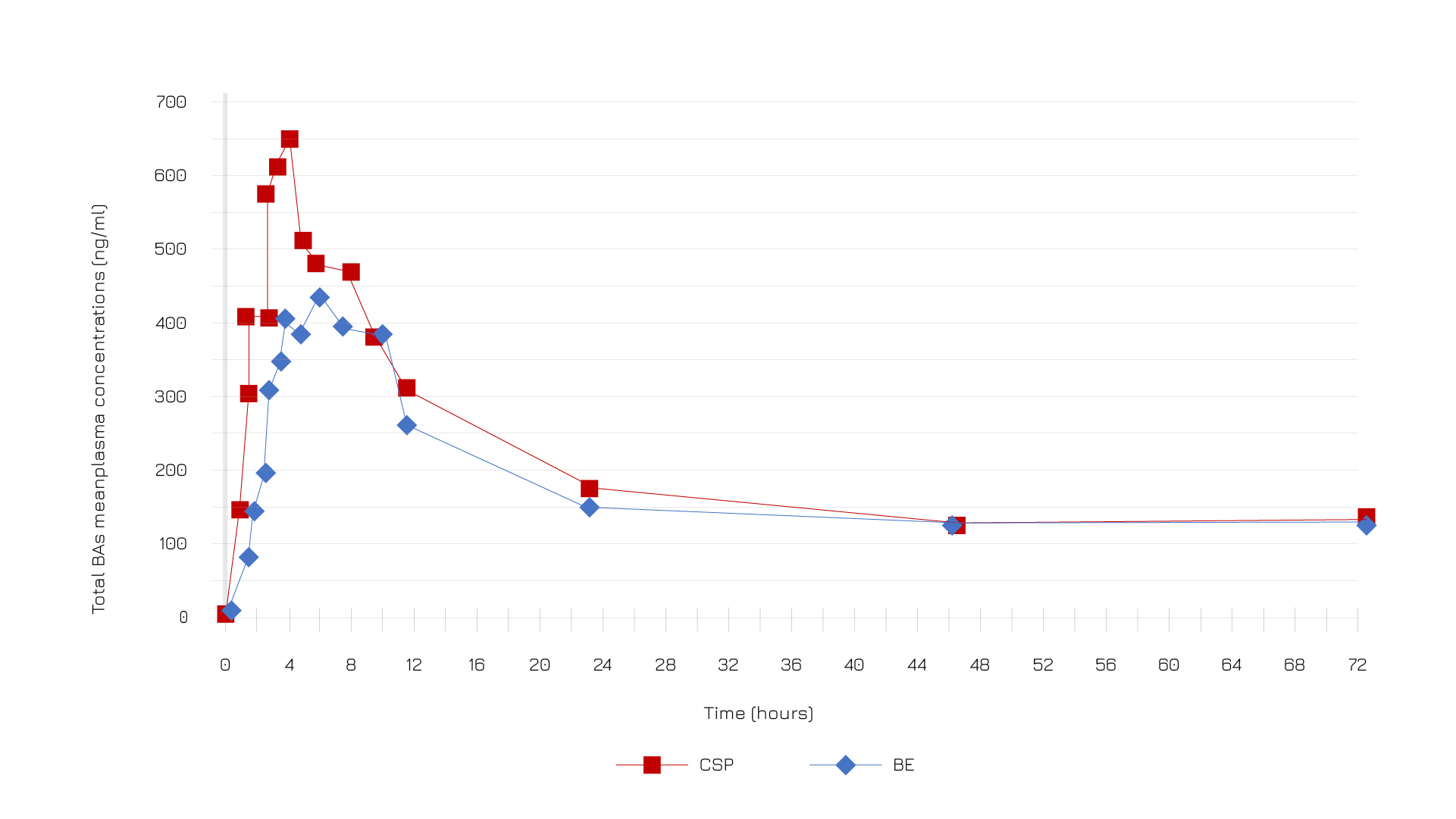
Casperome® delivers boswellic acids in average 3 times more efficiently and their peak concentration of boswellic acids are reached faster than in the baseline conditions than the basic extract (Figures 4 and 5).6
Figure 4: Total boswellic acids mean plasma concentrations (ng/mL) profile after administration of Casperome®(CSP) and not (BE).6
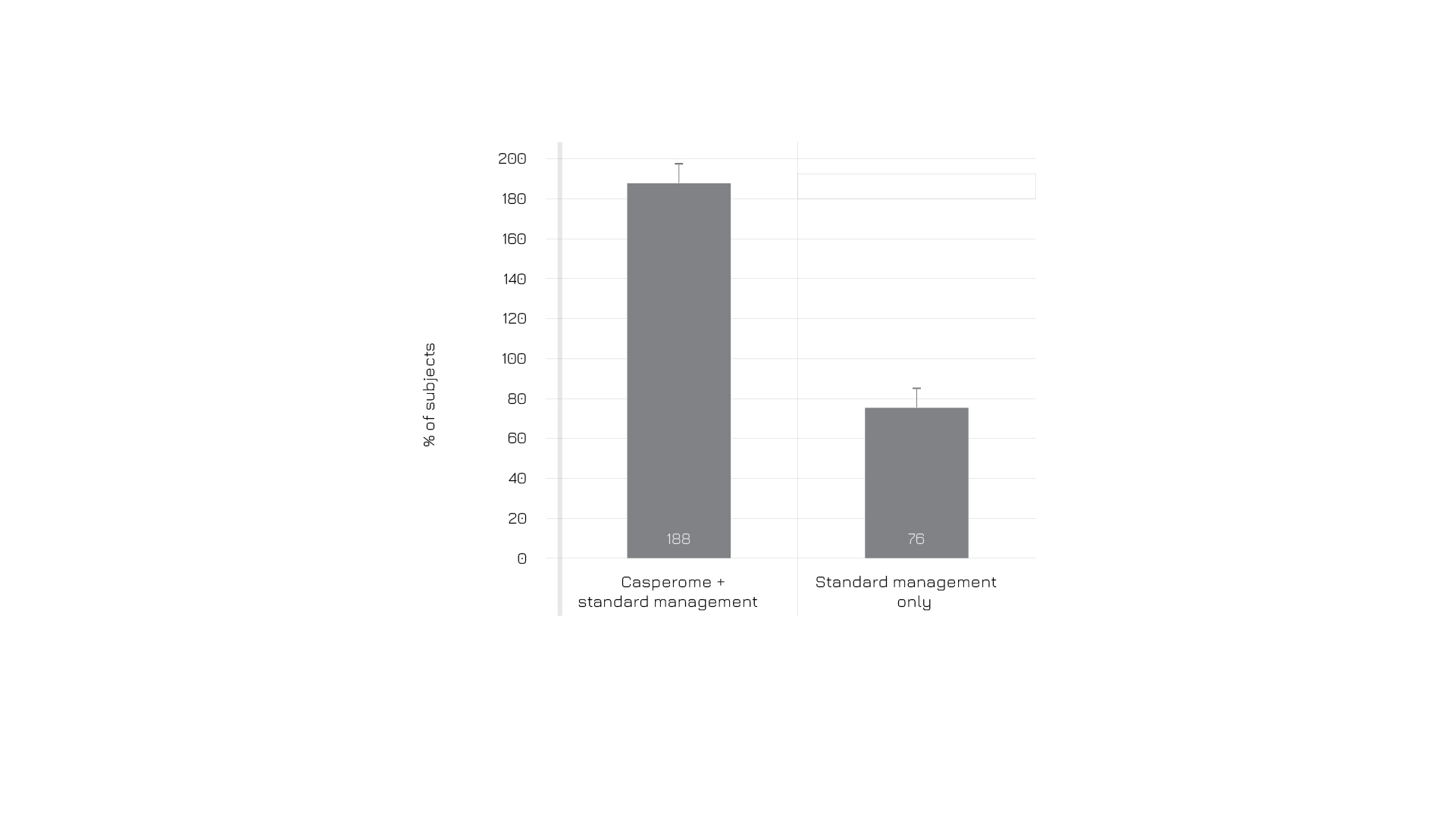
Figure 5: Proportion of subjects needing analgesic support (%), after a 4-week follow-up, either with Casperome®standard management or only standard management.6
BIBLIOGRAPHY
1. Giacosa, A et al. Nutrients 2022, 14, 1858.
2. Pellegrini L. et al.Eur Rev Med Pharmacol Sci 2016, 20: 2695-2700.
3. Riva, A., et al. Phytomedicine 23.12, 1375-1382 (2016).
4. Belcaro, G., et al. European Review for Medical and Pharmacological Sciences 21 (2017): 2249-2254.
5. Riva A, et al Minerva Gastroenterol Dietol. 2019 Mar;65(1):30-35.
6. Riva, A., et al.Eur Rev Med Pharmacol Sci 21.22, 5258-5263 (2017).
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.