浏览有关 Indena 产品、活动、公司信息等的所有文档。
前往查看Peer-reviewed science on Anthocran® Phytosome®
Anthocran® Phytosome® is the result of a continuous research and development process on cranberry extracts, that started with the identification of new biologically active metabolites and culminated with human studies on urinary tract health. 1,2,3
The ingredient fingerprint
New HPLC analysis allowed a complete profiling of the Anthocran® Phytosome® bioactive components, including PACs (proanthocyanidins) but also other natural occurring polyphenols typically found in the cranberry juice. In the end, results showed that Anthocran® Phytosome® maintains the same phytochemical profile of natural cranberry, thanks to the unique and careful industrial manufacturing processes.1
Moreover, thanks to a pharmacokinetic study, an innovative method allowed the identification of 42 urine metabolites providing new data for the understanding of the protective urinary tract activities of cranberry extracts. In particular, for the first time, valeric acids and valerolactons, well known PACs’ metabolites, have been identified in human urines after cranberry intake.2
Candida albicans and ut management
An ex-vivo study on human urines (13 healthy female volunteers) compared the effect of unformulated cranberry extract (unitary PACs dose=36 mg) to Anthocran® Phytosome® (unitary PACs dose = 9 mg) on Candida albicansproliferation in the urinary tract. Results showed that the new formulation matches the same performance of the standard extract even at lower concentration, thanks to the Phytosome® strategy that optimizes the absorption of of phytoactives’ compounds and their delivery to the target tissue (Figure 1).2
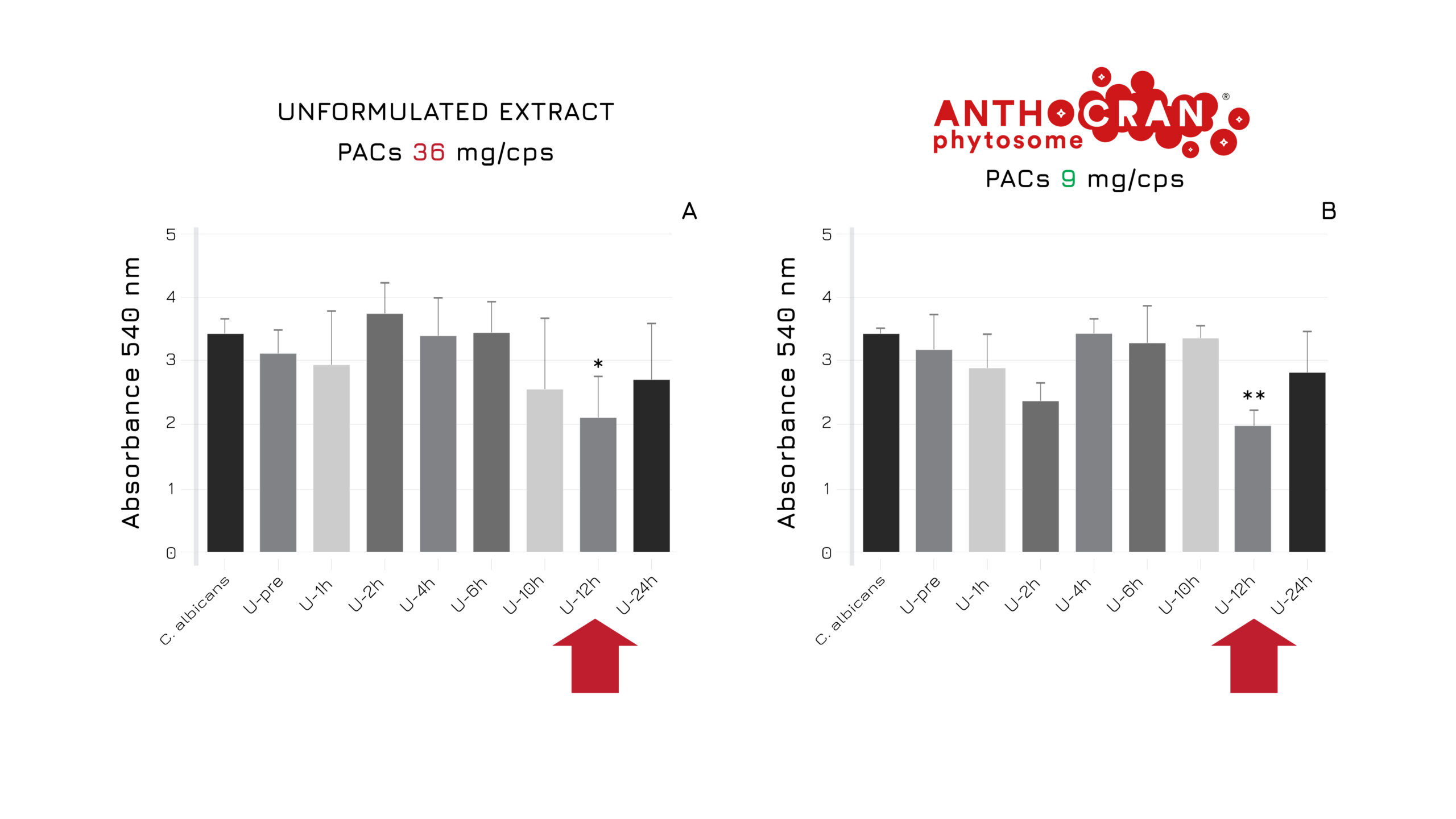
Figure 1: Unformulated extract vs Anthocran® Phytosome® activity on Candida albicansat different timesteps.2
Urinary tract protection: a human study
64 subjects with a non-complicated surgery requiring catheterization during a post-operative period and prone to UTIs, were included in a study to verify the actual benefit of the add-on treatment with Anthocran® Phytosome® (120 or 240 mg/die) compared to standard management. The 4 groups followed the respective supplementation for 4 weeks and were monitored for 3 months of foll0w-up. 3
Subjects belonging to Anthocran® Phytosome® groups experienced lower rate of urinary issues, measured with a validated VAS score. Furthermore, after 4 weeks no blood or traces of bacterial contamination were found in the correspondent samples, achieving the goal of zero total re-occurrence vs 17% and 18% in the two control groups.3
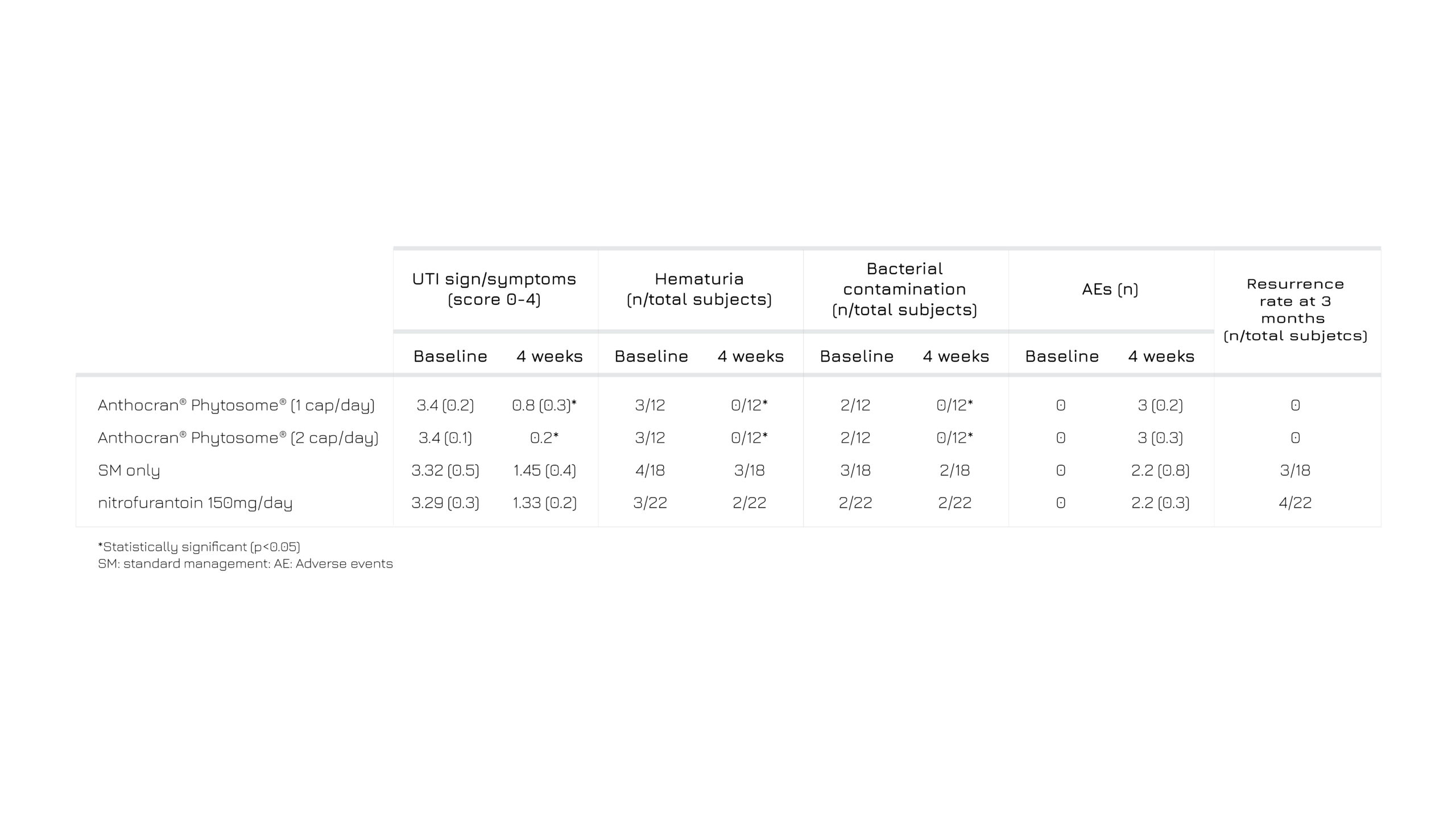
Table 1: Clinical efficacy and safety of Anthocran® Phytosome® vs SM.3
BIBLIOGRAPHY
1Indena internal data
2G.Baron et al; Biochemical Pharmacology, 173 113726, (2020)
3R.Cotellese et al; Journal of Dietary Supplements, E-pub (2021)

