Health Is
a Joint Project
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionWith more than 100 years of pharmaceutical excellence and a future-forward vision, Indena stands as the most reliable partner to design customized solutions for the development and manufacturing of natural derivatives and complex synthetic molecules.
Research and curiosity, technology and development, mastery and human genius and obsession for excellence: these are the key factors in Indena’s CDMO activity – and successful partnerships.
The expertise of our R&D team, covering analytical and process development, guarantees the kind of support and results only an expert partner with a strong foundation in science can provide.
As a privately owned company with a solid financial position, Indena has the vision and the capability to adopt new technologies, capitalizing on decades of know-how in the field of complex molecules. Our long-term strategy and vision have established us as a prominent and highly reliable strategic partner for a new generation of CDMO services.
Since 1921, Indena has been continuously expanding its range of high-quality services for the benefit of its partners. Our goal is to offer custom development and manufacturing solutions that retain the highest quality standards while guaranteeing enhanced flexibility – processing molecules that can be naturally derived (from botanical sources or microbial fermentation), semisynthetic, or synthetic.
We now manufacture 10 commercial and 12 clinical-phase HPAPIs, and continue to constantly innovate our suites for both HPAPIs and APIs to. Our plant in Settala, Italy currently handles substances with OEL as low as 1 ng/m3.
We always put a dedicated CDMO division, supported by a strong R&D team, at our partners’ service, and promptly implement custom solutions for specific projects requiring dedicated equipment.
The increasing number of oncology NCEs and antibody-drug conjugates, coupled with a growing focus on personalized medicine, is shaping the demand for CDMO services. The HPAPI market is undergoing a shift towards NCEs characterized by higher potency, growing molecular diversity and segmentation for specific populations.
Unlike newcomers in the field, Indena can count on an extensive know-how in safely handling HPAPIs, complemented by state-of-the-art equipment and installations. Within its long-term strategy, Indena has kept ahead of the curve by timely investing in specialized containment facilities that guarantee both employees and the external environment are safe from exposure, capitalizing on more than 30 years of know-how in high containment.
Indena’s fermentation department offers advanced capabilities in bio-transformation and secondary metabolite production using living cells. This expertise allows us to produce critical components in-house, such as toxins for Antibody Drug Conjugate (ADC) payloads, ensuring a fully integrated and independent supply chain.
With a deep mastery of GMP microbial fermentation and high-containment lines for downstream processing, we offer unmatched reliability and safety for precision fermentation and HPAPI development.
In an ever-evolving context where even a universally accepted definition for HPAPI has yet to be established, contract manufacturers must continuously invest in new specific facilities for this type of substance. Well aware of this, Indena has long been committed to advancing its CDMO services in this direction, investing in know-how, personnel, and hi-tech equipment.
Our latest expansion includes a state-of-the-art, 400-m2 R&D laboratory, equipped with 12 bench-top fume hoods, 2 walk-in fume hoods, a laminar flow fume hood, and 2 gloveboxes for up to 15 of the R&D scientists in our team of 70 experts. Additionally, a new industrial GMP line with reactors up to 10,000 liters enhances our capacity to produce and commercialize a wide range of regular APIs and HPAPIs.
With this proactive approach to innovation, we continue to stay ahead of market needs, offering unparalleled technological solutions and expertise to our CDMO clients.
The lower the OEL, the more potent the substance – and the greater the need for rigorous containment. Manufacturers must often adopt a conservative approach to ensure safety, particularly when dealing with early-stage NCEs, where data may be lacking to properly classify a substance as an HPAPI.
Indena, as a highly reliable Western European API producer, is equipped to handle substances with an OEL as low as 1 ng/m³, irrespective of their source. This includes toxin-based payloads for ADCs, supported by backward integration on fermentation and freeze-drying in high containment.
Indeed, having built a strong expertise in HPAPIs, Indena is now expanding its focus to ADCs – a promising area of targeted therapy that combines the specificity of monoclonal antibodies with the cytotoxic power of chemotherapeutic drugs, offering a more precise and potentially less toxic treatment approach.
In this context, in addition to producing payloads, Indena also manufactures linkers – key components in the assembly of ADCs – thus offering a more comprehensive and integrated solution to its partners.
We are already an established player in the ADC field, and we are continuously investing in additional capacity in a proactive way, anticipating the future demand of this rapidly evolving market.
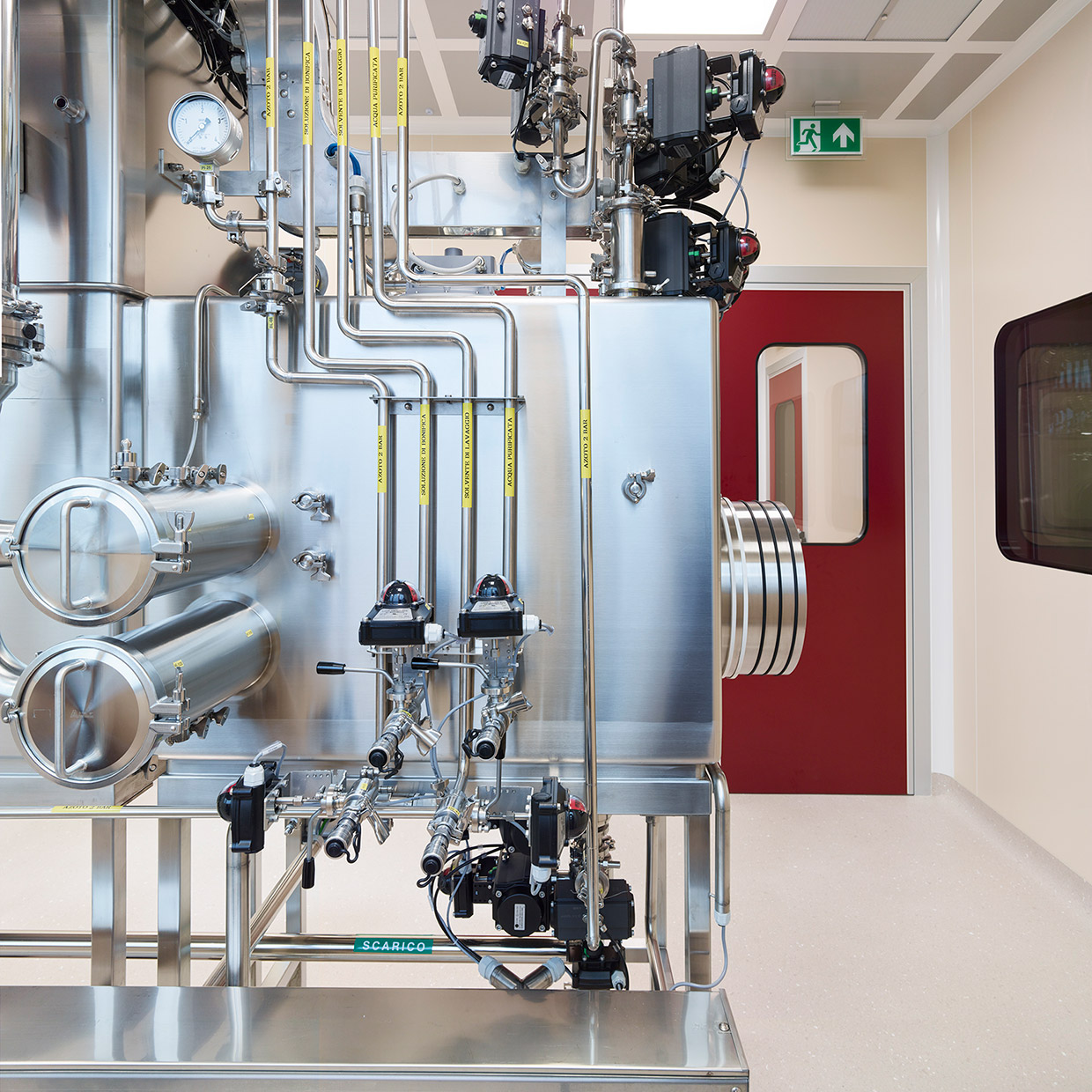
Indena’s main production site is equipped with a GMP plant featuring advanced capabilities, including 2 1,000-liter stainless steel reactors, 400- and 1,000-liter glass-lined reactors, a 250-liter hastelloy reactor for temperatures between -80 °C to +150 °C, a 500-liter chromatographic column, and a hastelloy centrifuge.
In 2024, the GMP plant was gradually expanded to increase its capacity for chemical synthesis and reactions 10-fold by early 2025, thus enabling larger-scale production of HPAPIs, from small molecules to OEB5, as well as a greater output of products that had been previously produced in other departments.
In addition, new advanced technologies for chemical synthesis and purification of APIs and HPAPIs have been introduced, including centrifugation under containment (reverse bag centrifuge), reactor loading under containment (glove boxes), isolation and drying on a filter dryer, and purification using 2,000 low/medium pressure columns (up to 9 bars). A newly acquired Biazzi hydrogenator, capable of operating at up to 10 barg, will further enhance production capabilities.
Substantial infrastructure upgrades in the LK2 plant in Settala have boosted its ability to handle HPAPIs, lowering maximum OEL from 20 to as little as 1 ng/m³. The site is already equipped with a small freeze dryer, with plans for a commercial-scale model in 2026.
Next year we also plan to integrate two new production lines: one with a 65-liter reactor and a 100-liter reactor, both inserted inside glove boxes, and a 40 cm diameter filter dryer in hastelloy C22; and the other with a freeze dryer with a capacity of 10 kg of ice in 24 hours, located inside a glove box equipped with a spray dryer.
All of these investments reinforce Indena’s leadership in HPAPI development and CDMO services, offering enhanced flexibility, safety, and scalability for projects at any stage of the pharmaceutical pipeline.
Our newly expanded high-containment laboratories set a new benchmark for HPAPI research and production, ensuring maximum safety and efficiency at every stage, from early research to industrial scale-up and mass production. All of our facilities meet the most stringent containment standards (OEL up to 1 ng/m³) and enable secure segregation between GMP manufacturing and R&D, allowing for precise development regardless of volumes.
Equipped with large glove boxes, high-containment hoods, and specialized systems for development and analysis – including crystallization, chromatography, and spray drying – our labs empower a new generation of highly skilled researchers, reinforcing our role as a leading CDMO partner even for the most complex projects.
On top of our focus on HPAPIs, we have leading expertise in the pre-formulation of poorly soluble APIs. Our combination of multiple, highly specialized technologies in one site allows us to optimize the supply chain: for example, we are able to carry out the synthesis of an API and the subsequent spray drying step using organic solvents, also in the presence of excipients to obtain a pre-formulated drug product. Indena is equipped with development- and commercial-scale spray dryers to provide clinical and commercial volumes. These activities are fully supported by our analytical team. In addition to spray drying, we have proprietary technologies to obtain solid amorphous dispersion (such as Indena Phytosome®) available.
Indena has recently opened a multipurpose pilot GMP plant for productions of clinical and commercial small-scale; we are also currently implementing additional synthetic capacity, to anticipate future market demand.
In the past few years, Indena has upgraded its fermentation capacity, in a multipurpose plant that can also handle toxic compounds. Currently, the plant is equipped with 100-, 1,000- and 20,000-liter fermenters. A dedicated biotech team now supports development and scale-up activities, assisting the technology transfer to production.
At Indena, we see ourselves as strategic partners, going beyond the traditional client-supplier relationship by fully committing to the development of new HPAPIs and APIs, from early clinical stages through to commercial-scale manufacturing. This commitment is supported by continuous investments in innovative technologies and services, ensuring we meet and exceed our clients’ expectations.
However, the true strength of our CDMO services lies in our people. At Indena, we combine the lifelong professional experience of seasoned experts with the fresh perspectives and enthusiasm of young scientists. This unique blend of talent ensures that we deliver exceptional results, backed by a corporate culture rooted in quality and rigorous HSE (Health, Safety, and Environment) policies.
Our strong regulatory department is a cornerstone of our ability to deliver high-quality services to clients, navigating complex and ever-evolving global health regulations.
By maintaining ongoing interactions with key authorities such as the FDA (U.S. Food and Drug Administration), EMA (European Medicines Agency), AIFA (Italian Medicines Agency), ANSN (French National Agency for Medicines and Health Products Safety), and PDMA (Pharmaceuticals and Medical Devices Agency in Japan), our experts ensure that all aspects of our operations are not only safe and efficient but also fully compliant with the highest industry standards.
Sustainability is at the heart of all our operations, not only to benefit the environment but also to ensure business continuity through efficient resource management and compliance with EU climate change policies.
Our commitment to reducing fossil fuel consumption and enhancing energy efficiency is evident across our facilities, equipped with over 4 MW of photovoltaic systems, projected to generate 4,000,000 kWh of clean energy annually. We also invest in high-efficiency chillers, nitrogen production systems, and heat pumps to optimize water and heat usage, contributing to our broader sustainability goals.
As further evidence of our environmental stewardship, our portfolio includes innovative, sustainable products such as plant-based squalene, produced from Amaranthus seeds instead of shark liver; resiniferatoxin, derived from the sustainable cultivation of the Euphorbia plant; and cytisine, supported by a controlled, certified, and fully traced supply chain.
These efforts, along with our EcoVadis Bronze Medal certification, position Indena as a leader in sustainability, ensuring long-term reliability and success for our clients.
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.