Relissa™
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionPeer-reviewed science on Relissa™
A randomized, placebo-controlled study was conducted on 100 subjects experiencing mild sleep problems (PSQI score >5) or individuals experiencing mild to moderate mental stress. Mental and sleep health were measured at baseline and after 3 weeks of RelissaTM supplementation (200 mg) (n=52) or placebo (n=48) twice a day.1
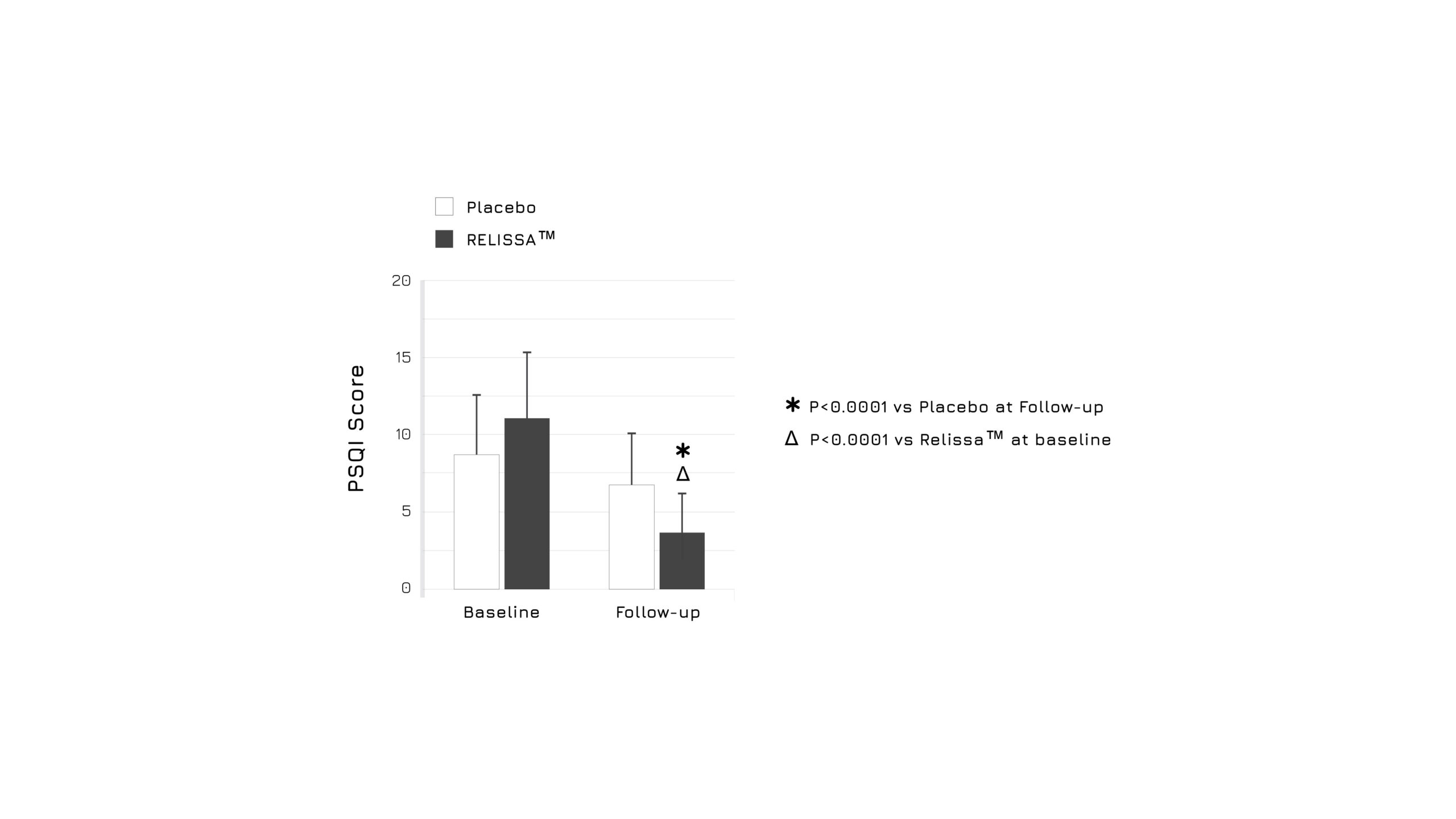
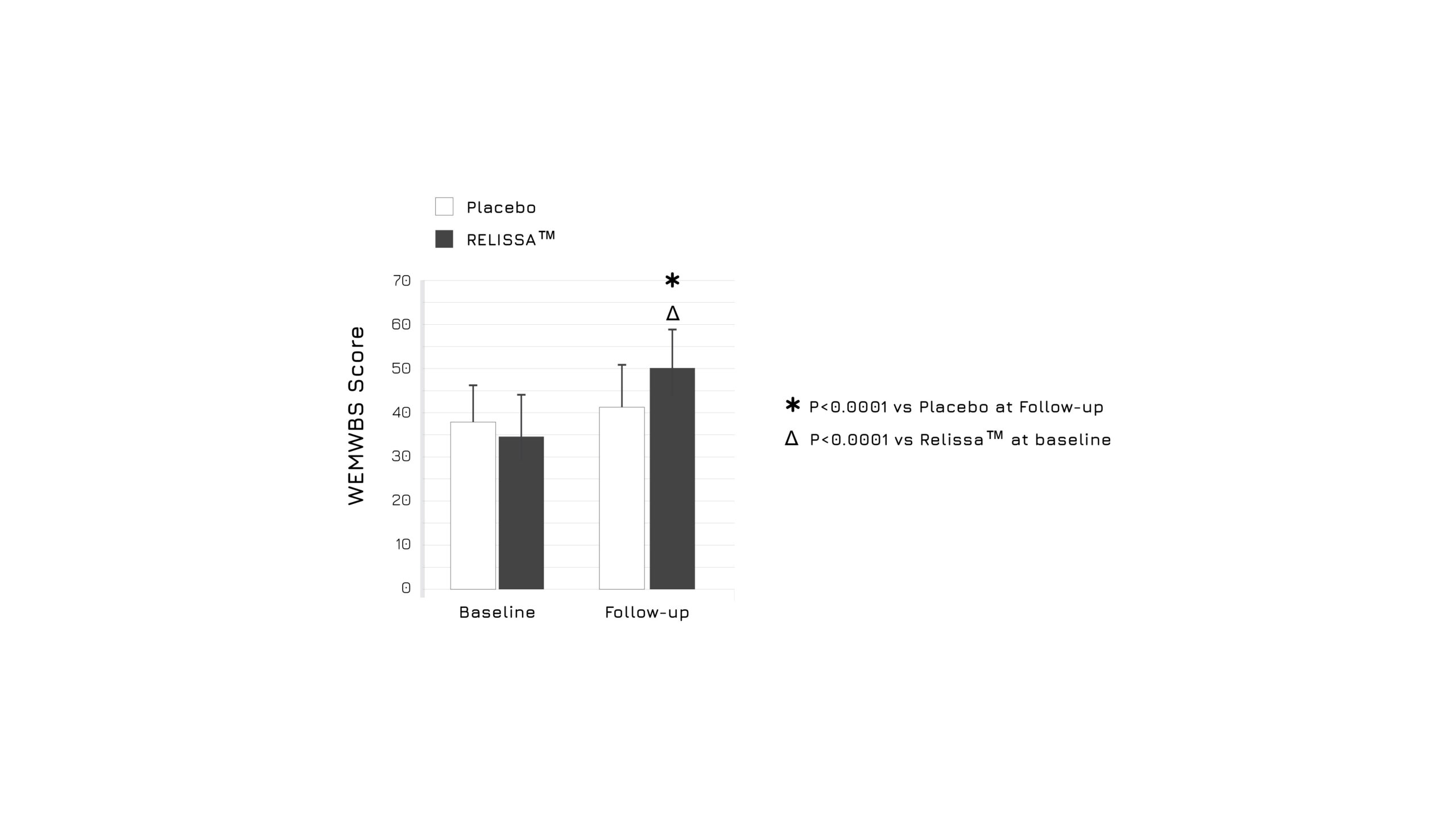
After 3 weeks of administration, the study showed a significant increase in quality of sleep in subjects belonging to the intervention group when compared to baseline levels and to the placebo group (p<0.0001) (Figure 1). Moreover, authors highlighted a relevant beneficial effect in mental wellbeing (WEMWBS Score) (p<0.0001) (Figure 2), as well as a reduction on DASS Score indicators (p<0.0001).1
Figure 1. Graphical representation of the sleep health variation in placebo and supplemented groups after 3 weeks. The perceived sleep quality and disturbances were assessed using the PSQI Score.
Figure 2. Graphical representation of the mental wellbeing modulation in placebo and supplemented groups after 3 weeks. Measurements were assessed using the Warwick Edinburgh Mental Wellbeing Scale (WEMWBS).
Peer-reviewed science on Relissa™
With the growing prevalence of smartwatches and their widespread use, individuals can now directly experience and monitor the beneficial effects of a supplement in real-time. These wearable devices provide an accessible platform for users to track key health indicators, offering valuable insights on well-being through continuous personal monitoring.
Given that, in a recent double-blind placebo controlled human study2 the effects of RelissaTM on improving sleep quality has been evaluated with the support of a wearable device, particularly focusing on its ability to naturally enhance sleep without the use of medications.
The study aimed to assess key sleep parameters, such as insomnia symptoms, time spent in deep sleep (SWS phase), and REM sleep, as well as participants' subjective perception of sleep quality.
By comparing RelissaTM to a placebo, the researchers sought to determine whether this natural ingredient could offer a safe and effective alternative for improving sleep patterns and reducing insomnia symptoms. The study demonstrated significant improvements in sleep quality.
Participants using RelissaTM experienced a reduction in the Insomnia Severity Index (ISI) score of 2.9 points (p = 0.003), with an average of 6.8 ± 4.1 compared to 9.7 ± 3.7 in the placebo group.
Additionally, there was a 15% increase in the duration of deep sleep (SWS phase) and a 10% reduction in REM sleep, both statistically significant (p < 0.05).
Slow-wave sleep has been extensively studied as a key predictor of sleep quality, being considered the most restorative sleep stage, promoting good cognitive performance during the day; balanced cycle between REM and SWS is necessary for individual health.
Furthermore, 87% of participants reported improved sleep quality while using RelissaTM, compared to only 30% in the placebo group (p = 0.0003).
Peer-reviewed science on Relissa™
A preclinical study3 on Relissa™ aimed to evaluate its antioxidant and neuroprotective effects, particularly investigating how well it could support physiological GABA pathway and protect brain cells from damage caused by oxidative stress. The researchers compared Relissa™ to a traditional unformulated lemon balm extract to see if can offer better performance.
Relissa™ exhibited stronger inhibitory activity on the GABA-t enzyme (gamma-aminobutyric acid transaminase), compared to the poor inhibition by the unformulated dry extract. This is particularly significant because GABA is a fundamental neurotransmitter in the central nervous system (CNS), and low GABA levels are associated with anxious states and various mental disorders.
The evaluation of antioxidant activity in SH-SY5Y neuronal cells, a cellular model for studying neurodegeneration, showed that RELISSA™ provides significantly greater protection against H₂O₂-induced oxidative stress compared to the traditional unformulated extract, suggesting a potential neuroprotective effect.
Similarly, in cell-free systems, the melissa Indena Phytosome™ demonstrated superior antioxidant effects compared to the traditional extract, significantly reducing ROS production.
These in vitro results demonstrate that Relissa™ may contribute significantly to the management of emotional distress and related conditions, such as anxiety and sleep disorders, thanks to its neuroprotective and antioxidant properties, which can combat oxidative stress-induced damage.
BIBLIOGRAPHY
1. Bano A, et al. (2023) Front. Pharmacol. 14:1250560. doi: 10.3389/fphar.2023.1250560.
2. Di Pierro F., et al. Nutrients 16.23 (2024): 4199.
3. Kara M., et al. (2024) Frontiers in Molecular Biosciences 11: 1359177 Neurosci 2017.
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.